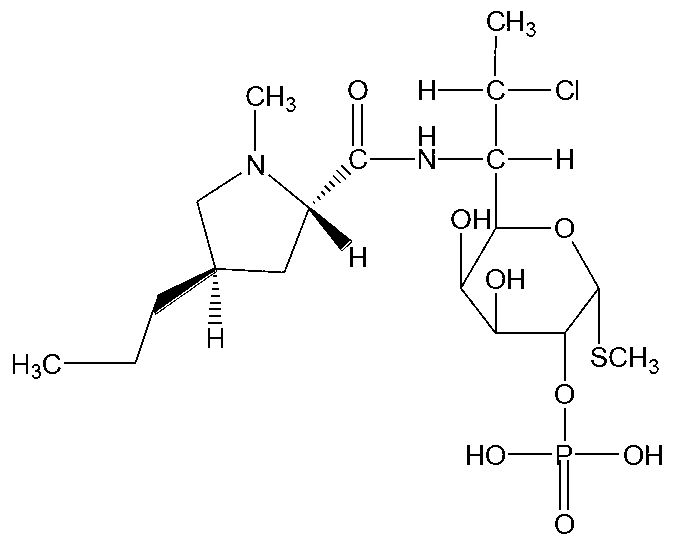
Clindamycin phosphate compound and preparation method and pharmaceutical composition thereof
A technology of clindamycin phosphate and compounds, which is applied to the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problems of unsuitability for clinical application and potential safety hazards, and achieve the reduction of stability and good Effects of stability and concentration of particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: Preparation of Clindamycin Phosphate Compound
[0046] (1) Prepare 5L of a saturated solution of clindamycin phosphate solid at 45°C;
[0047] (2) Prepare 25L of mixed organic solvent of ethyl acetate, acetone and ether; the volume ratio of ethyl acetate, acetone and ether in the mixed organic solvent is 1:2:3;
[0048] (3) Cool the organic solvent to 0°C, and add a saturated aqueous solution of clindamycin phosphate to the organic solvent at a uniform speed under the condition of a stirring speed of 720 rpm, and continue stirring after the addition, and the stirring speed is 360 rpm Minutes; stop stirring after cooling down to 0°C; stand for crystal growth for 8 hours; filter after obtaining crystals, wash with absolute ethanol, and vacuum dry for 4 hours to obtain clindamycin phosphate compound; saturated aqueous solution of clindamycin phosphate The addition rate was 2.5 liters / hour.
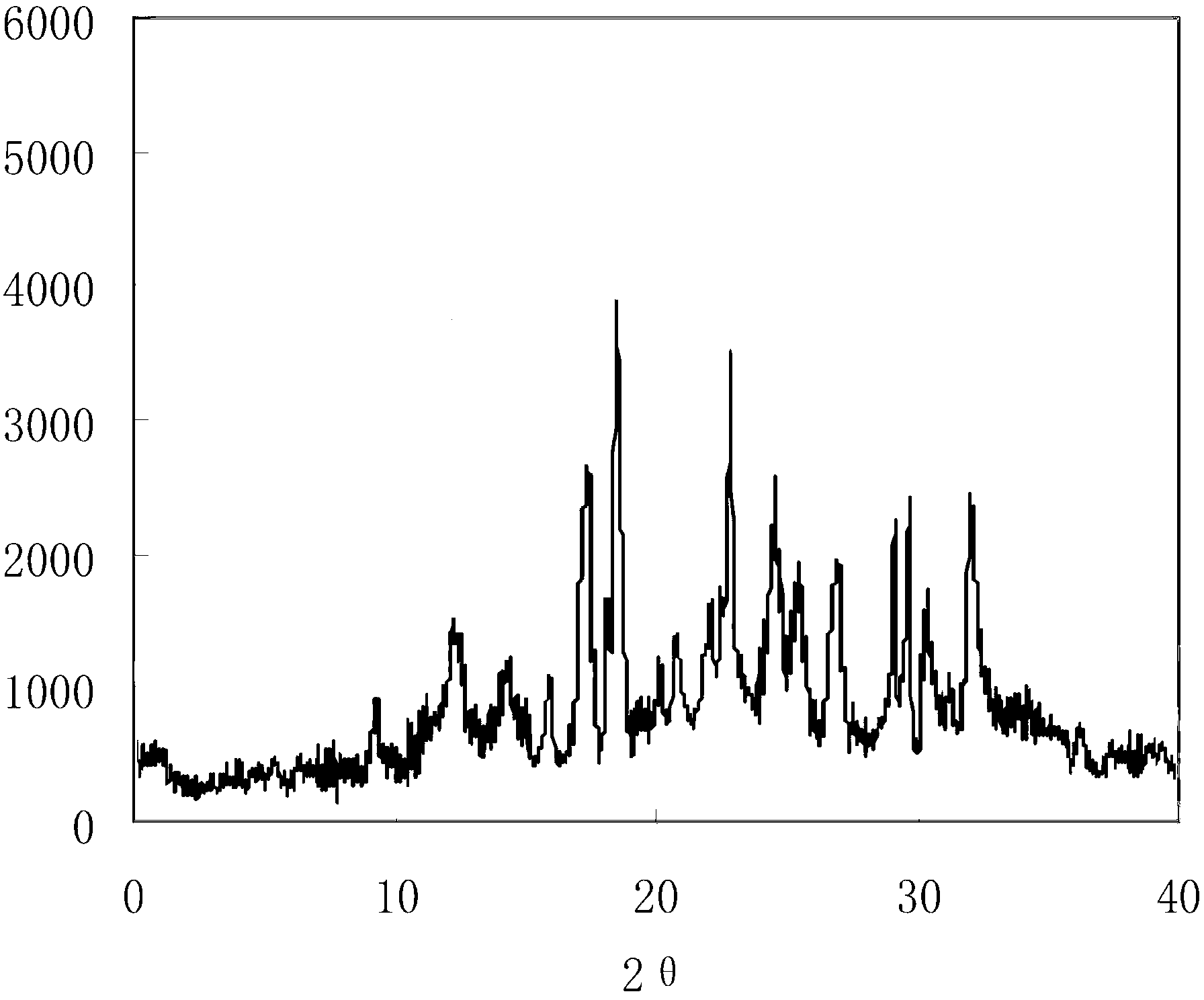
[0049] Prepared clindamycin phosphate obtained by Cu-Kα ray measur...
Embodiment 2
[0050] Embodiment 2: Preparation of clindamycin phosphate compound
[0051] (1) Prepare 5L of a saturated solution of clindamycin phosphate solid at 58°C;
[0052] (2) Prepare 50L of mixed organic solvent of ethyl acetate, acetone and ether; the volume ratio of ethyl acetate, acetone and ether in the mixed organic solvent is 1:2:2;
[0053] (3) Cool the organic solvent to 5°C, and add a saturated aqueous solution of clindamycin phosphate to the organic solvent at a uniform speed under the condition of a stirring speed of 360 rpm. Minutes; stop stirring after cooling down to 0°C; let stand to grow crystals for 8 hours; filter after obtaining crystals, wash with absolute ethanol, and dry in vacuum for 4 hours to obtain clindamycin phosphate compound, saturated aqueous solution of clindamycin phosphate The rate of addition is 5 liters / hour.
[0054] Prepared clindamycin phosphate obtained by Cu-Kα ray measurement X-ray powder diffraction pattern as figure 1 As shown; the purit...
Embodiment 3
[0055] Embodiment 3: Preparation of Clindamycin Phosphate Compound
[0056] (1) Prepare 5L of a saturated solution of clindamycin phosphate solid at 50°C;
[0057] (2) Prepare 40L of mixed organic solvent of ethyl acetate, acetone and ether; the volume ratio of ethyl acetate, acetone and ether in the mixed organic solvent is 1:3:3;
[0058] (3) Cool the organic solvent to 2°C, and add a saturated aqueous solution of clindamycin phosphate to the organic solvent at a uniform speed under the condition of a stirring speed of 720 rpm, and continue stirring after the addition is completed, and the stirring speed is 360 rpm Minutes; stop stirring after cooling down to 1°C; stand still for 8 hours to grow crystals; filter after obtaining crystals, wash with absolute ethanol, and vacuum dry for 4 hours to obtain clindamycin phosphate compound; saturated aqueous solution of clindamycin phosphate The adding speed is: 5 liters / hour.
[0059] Prepared clindamycin phosphate obtained by Cu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Master granularity | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Distribution width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com