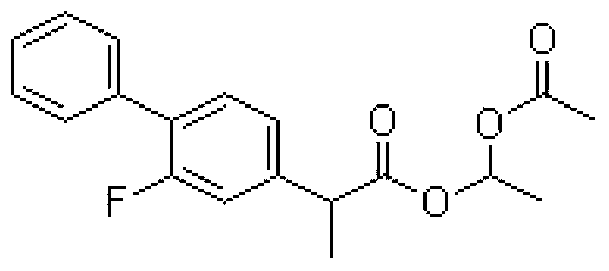
S (+) -flurbiprofen axetil emulsion for injection
A technology of flurbiprofen axetil and flurbiprofen, applied in the field of S(+)-flurbiprofen axetil injection emulsion, which can solve problems such as increased gastrointestinal side effects and lack of cyclooxygenase inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Preparation of S(+)-flurbiprofen axetil injection emulsion
[0016] Preheat 50g of soybean oil for injection to 60 degrees Celsius, add 5g of S(+)-flurbiprofen axetil, dissolve, and then disperse 6g of egg yolk lecithin in it to obtain an oil phase; mix 11.25g of glycerin for injection and 0.25g Add disodium hydrogen phosphate to water for injection, and preheat to 60 degrees Celsius to obtain the water phase; disperse the oil phase in the water phase, stir at 8000rpm for 20 minutes to make rough milk; homogenize the thick milk under 700bar high pressure for 6 times, Obtain essence milk; adjust the pH of essence milk to 6.5 with citric acid, filter through a 0.8 micron filter membrane, fill in 5ml ampoules, and sterilize at 121 degrees Celsius for 15 minutes to obtain the finished product. The specification of the obtained emulsion for injection is 50mg / 5ml.
[0017] The following pharmacological studies were carried out using the emulsion for injection prepared in the...
Embodiment 2
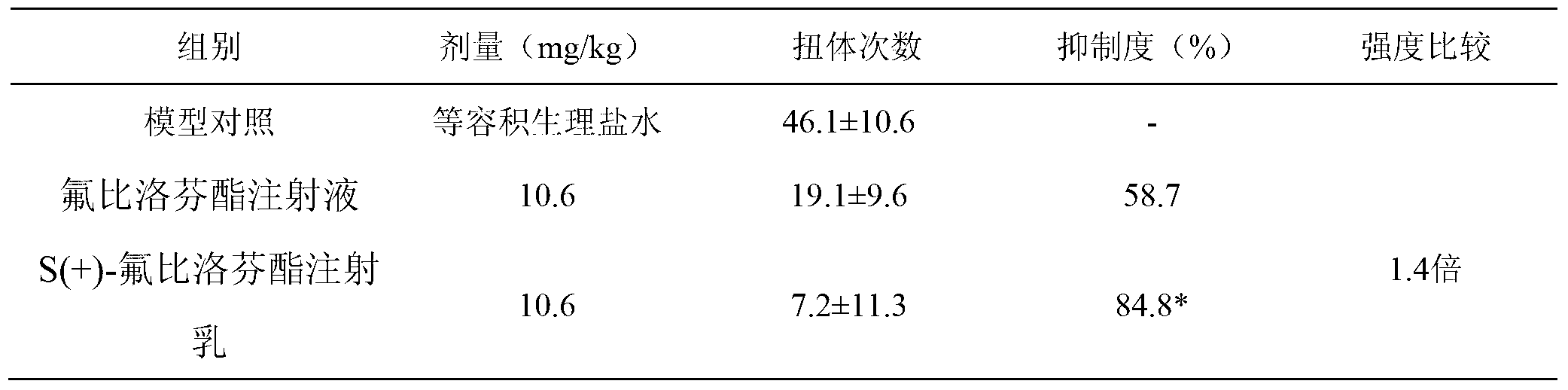
[0019] Analgesic effect of S(+)-flurbiprofen axetil injection milk
[0020] 30 KM mice, half male and half female, weighing 18-22 g, were randomly divided into 3 groups, 10 mice in each group. They are model control, flurbiprofen axetil injection group, and S(+)-flurbiprofen axetil injection milk group (please refer to the declared patent 201210153560.1 for the preparation prescription and process). All groups were administered by injection. After 10 minutes of administration, each mouse was intraperitoneally injected with 0.6% acetic acid solution 0.1mL / 10g. Observe the number of writhing reactions in mice within 15 minutes, calculate the analgesic inhibition rate, and compare between groups. The results showed that, compared with the control group, each treatment group had obvious analgesic effect, and the effect of S(+)-flurbiprofen axetil injection milk group was more significant. see table 1
[0021] Table 1 Inhibitory effect of drugs on acetic acid writhing response i...
Embodiment 3
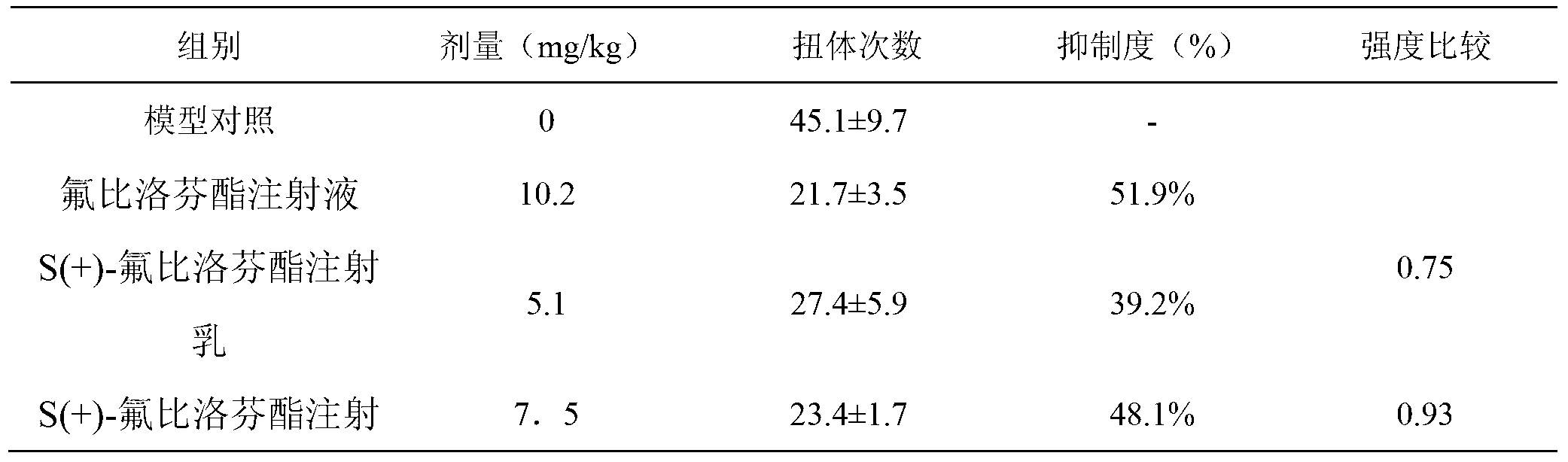
[0025] Pharmacokinetics of S(+)-flurbiprofen axetil injection milk
[0026] Based on the findings in Example 1, the pain intensity of S(+)-flurbiprofen axetil injection emulsion is about 1.4 times that of flurbiprofen axetil injection. In other words, it is expected that a smaller dose of S(+)-flurbiprofen axetil injection emulsion can achieve the same pain effect as flurbiprofen axetil injection. Therefore, in the follow-up study, the dose of S(+)-flurbiprofen axetil injection emulsion was reduced to 50% and 75% of the above study.
[0027] 40 KM mice, half male and half female, weighing 18-22 g, were randomly divided into 4 groups, 10 mice in each group. Each group was administered by injection, and the doses were 0 (vehicle), 10 mg / kg (flurbiprofen axetil injection), 5 and 7.5 mg / kg (S(+)-flurbiprofen axetil injection emulsion), and After 10 minutes of drug administration, each mouse was intraperitoneally injected with 0.6% acetic acid solution 0.1 mL / 10 g. Observe the n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com