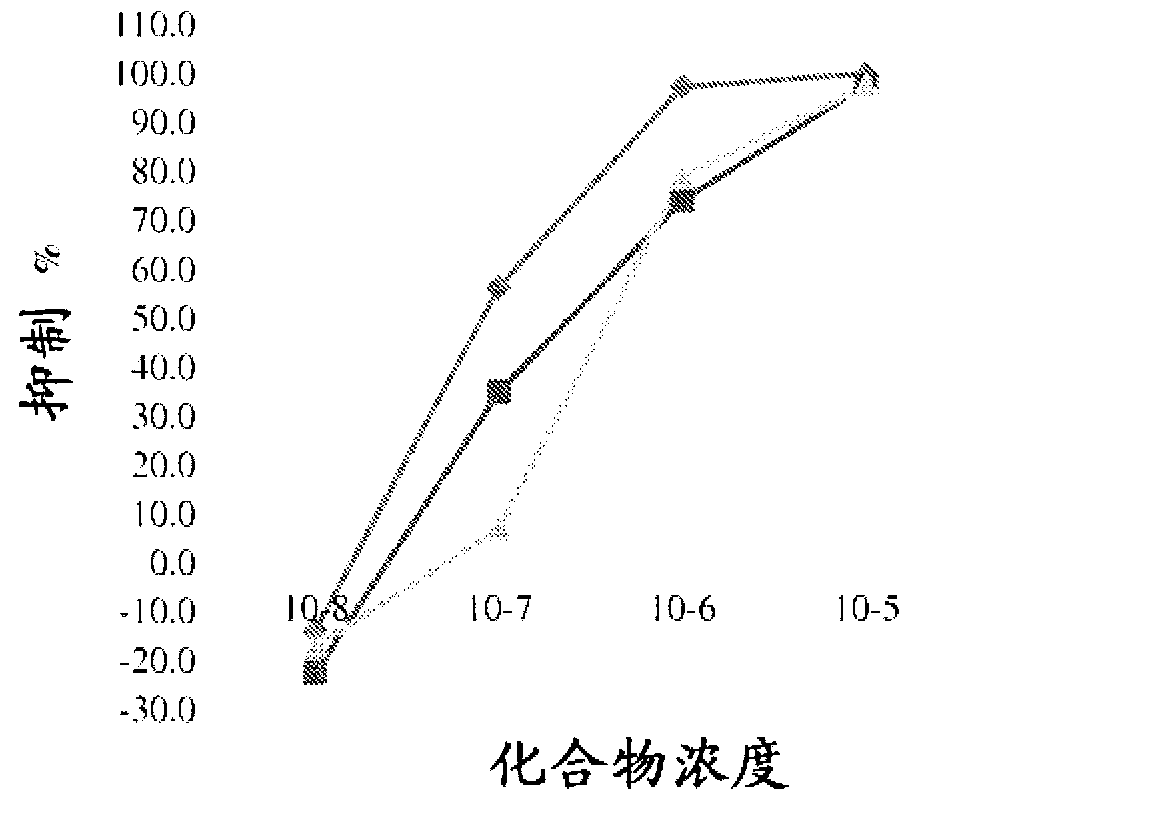
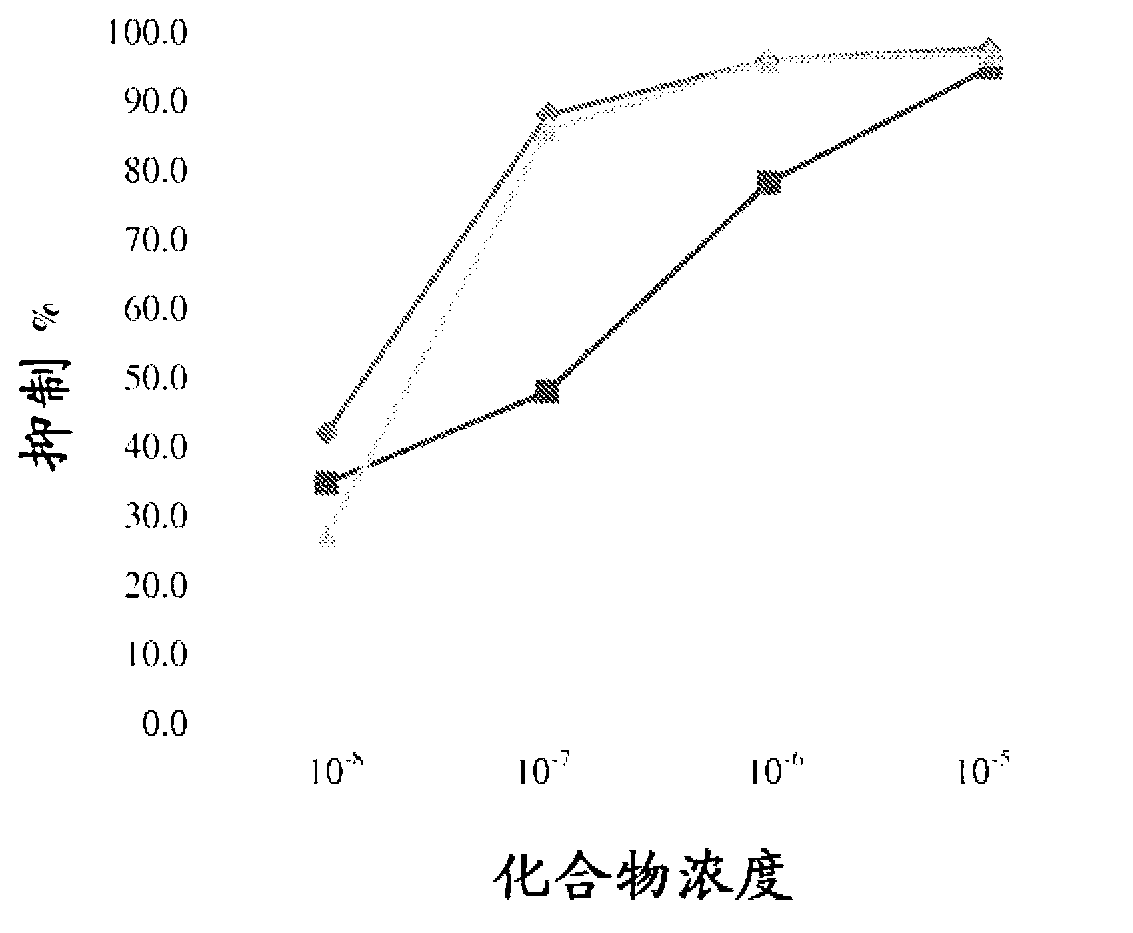
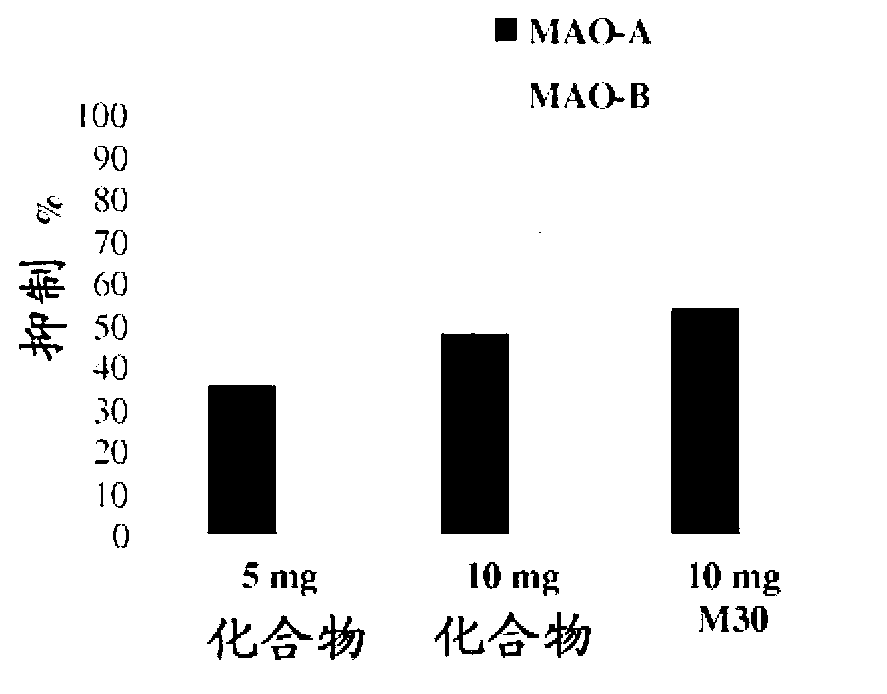
Neuroprotective and neuro-restorative iron chelators and monoamine oxidase inhibitors and uses thereof
A compound, alkoxy technology, applied in the field of novel multifunctional neuroprotective compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0123] Example 1. Synthesis of 5-[(methyl-2-propyn-1-ylamino)methyl]-8-quinolinol (M30)
[0124] The M30 described herein was synthesized as follows:
[0125]
[0126] Reagents: (a) HCl (32%), HCHO (37%), 0~rt; (b) N-methylpropargylamine, (Me 2 CH) 2 NEt, CHCl 3 , rt.
[0127] In water at 0°C, 14.6 g (0.1 mol) of 8-quinolinol, 16 mL of 32% HCl, and 16 mL (0.1 mL) of 37% formaldehyde were mixed with hydrogen chloride gas for 6 h. The solution was allowed to stand at room temperature for 2 hours without stirring. The resulting yellow solid was collected on a filter, washed with 90% ethanol and dried under vacuum to give 5-chloromethyl-8-quinolinol HCl salt A (19.0 g, 98%): 1 H NMR (250MHz, CDCl 3 , δ) 5.32 (s, 2H), 7.53 (m, 1H), 7.85 (m, 2H), 8.12 (m, 1H), 9.12 (m, 1H), 9.28 (m, 1H).
[0128] in 50mL CHCl 3 , 5-chloromethyl-8-quinolinol HCl salt A (2.707 g, 11.8 mmol) and diisopropylethylamine (DIPEA; 2.1 mL, 20.4 mmol, 2 eq) were mixed, and N- Methyl-N-propargylamin...
Embodiment 2
[0129] Example 2. Synthesis of Ester M30
[0130] The ester M30 described herein was synthesized as follows:
[0131]
[0132]A solution of 1 eq M30 and 1 eq triethylamine (TEA) in tetrahydrofuran (THF) under nitrogen atmosphere by dropwise addition of 1 eq corresponding acid chloride in THF (eg, R=-CH3, acetyl chloride) solution treatment. After completion (TLC or HPLC analysis), the mixture was concentrated in vacuo and the residue was partitioned between ethyl acetate and water. The organic phase was dried over magnesium sulfate and evaporated in vacuo. The crude product was purified by crystallization or column chromatography.
[0133] In this way, the following esters can be prepared, having at the 8-position of the group: -OCOCH 3 , -OCOCH 2 CH 3 , -OCOCH(CH 3 )CH 2 CH 2 CH 3 , -OCOCH 2 CH(CH 3 ) 2 , -OCOCH 2 Cl, -OCOCH 2 OCH 3 , -OCOCH 2 CH 2 OCH 2 CH 3 , -OCOCH=CH 2 , -OCOC(CH 3 )=CH 2 , -OCOCH=CH(CH 3 ), -OCOCH=CHPh, -OCOCH 2 CH 2 CH=CH 2...
Embodiment 3
[0134] Example 3. Synthesis of amino acid esters of M30
[0135] The M30 amino acid esters described herein were synthesized as follows:
[0136]
[0137] Using the procedure of Song, et al. (J.Med.Chem., 2005, 48, 1274-1277), t-Butoxycarbonyl (Boc) protected amino acid (5eq), N,N'-cyclohexylcarbodioxide imine (DCC; 5eq) and 4-dimethylaminopyridine (DMAP: 0.5eq) were reacted with M30 (1eq) in dry 4-dimethylaminopyridine (DMAP: 0.5eq) at room temperature 24h. The reaction was filtered and the DMF was vacuum dried. The residue was dissolved in ethyl acetate and washed with water and brine. The organic layer was dried over magnesium sulfate and concentrated in vacuo. Purification by column chromatography. In the case of aspartic acid and glutamic acid, the t-butyl protected beta and gamma carboxylic acids, respectively, were utilized. The purified product was treated with trifluoroacetic acid (TFA): dichloromethane (DCM) [1:1]. After 4 h, the solvent was removed in vacu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com