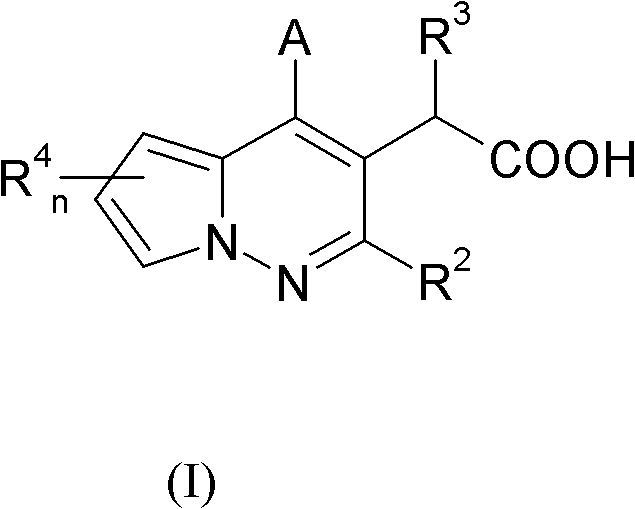
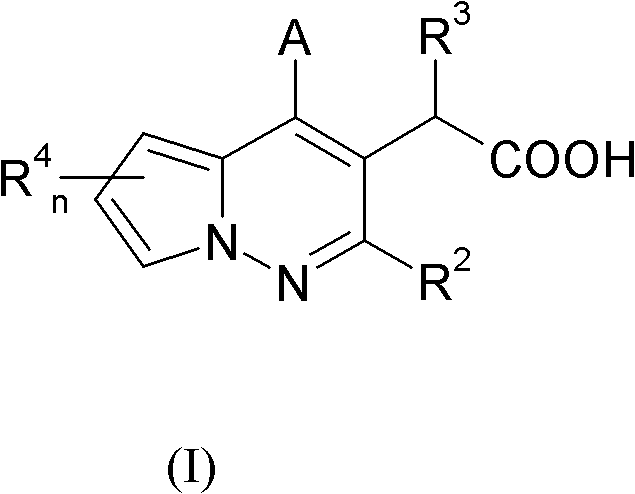
Pyrrolopyridazine compounds with antiviral properties
A compound, alkyl technology, applied in the field of HIV treatment, HIV replication inhibitor, integrase allosteric inhibitor, can solve crystal form stability, solubility lipophilicity, CYP inhibition liver microsome stability and plasma stability sexual insufficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0183] Example 1: Synthesis using pyrrolo[1,2-b]pyridazine derivatives.
[0184]
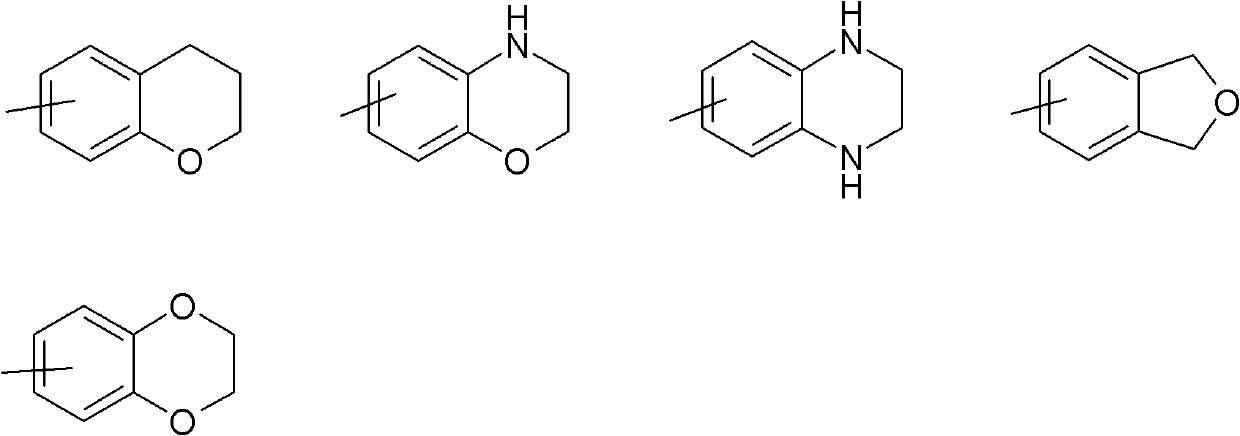
[0185] A compound of formula (I), wherein R 3 Yes - O(C 1 -C 6 )alkyl. More specifically, -O tert-butyl, R 2 , R 4 A is a compound of formula (I) as defined herein and can be prepared according to Scheme 1.
[0186] Scheme 1 discloses a method for synthesizing compounds of formula (I).
[0187] A general method for the synthesis of pyrrolo[1,2-b]pyridazine derivatives, starting with a 1-aminopyrrole derivative, eg 1a. The original method for the synthesis of pyrrolo[1,2-b]pyridazines from 1-aminopyrrole and β-dicarbonyl compounds, see Flitsch and Kramer (Flitsch, W.; Kramer, U. Tetrahedron Lett. 1968, 1479 and Flitsch, W .; Kramer, U. Liebigs Ann. Chem. 1970, 735, 35).
[0188] Condensation of 1-aminopyrrole (1a) and diethyl (ethoxymethylene)malonate (1b) in a suitable solvent, optionally heated and catalyzed with acids such as toluenesulfonic acid, will form pyrrolo[1,2 -b] pyridazi...
Embodiment 2
[0195] Example 2 Another synthetic method of pyrrolopyridazine derivatives.
[0196]
[0197] Scheme 2 discloses an alternative method for the synthesis of compounds of formula (I). A Condensation of 1-aminopyrrole derivative (2a) with β-dicarbonyl compound 2b in a suitable solvent, optionally acid catalyzed or heated, to obtain 2c.
[0198] Compound 2c and 4-acetamidobenzenesulfonyl azide in anhydrous acetonitrile were treated with DBU and stirred to give azide 2d (conditions were similar to those described in Hahn et al. J. Organometallic Chem. 2004, 689, 2662).
[0199] Heating, or optionally microwave treatment of 2d with rhodium(II) acetate dimer and isopropanol yields 2e.
[0200] Carry out saponification with lithium hydroxide or sodium hydroxide of 2e, generate the compound of structural formula (I).
[0201] Those skilled in the art will recognize that compounds of formula (I) have a chiral center. Those skilled in the art will recognize that there are many enan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com