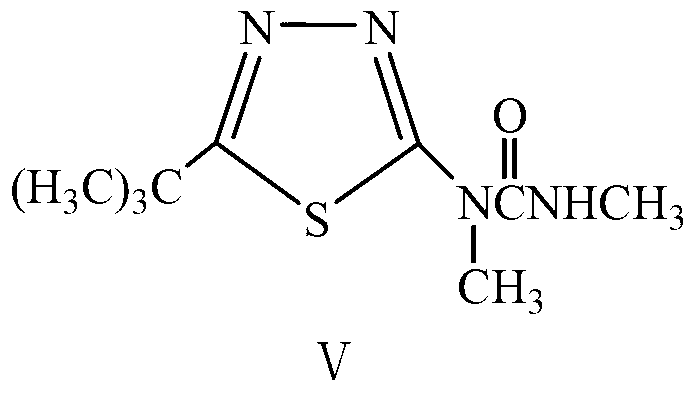
Synthesis method of key intermediate of tebuthiuron, namely 2-methylamino-5-tert-butyl-1, 3, 4-thiadiazole
A technology of terbutazone and methylamino, which is applied in the field of compound preparation, can solve the problems of not conforming to the development direction of green chemistry, difficult wastewater treatment, serious environmental pollution, etc., and achieves high atom economy and high yield , the effect of less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 10.5 g (0.1 mol) of 99% 4-methyl-3-thiosemicarbazide (I) and 30 mL of 1,2-dichloroethane to a 100 mL reaction flask, heat it to 80°C, turn off the heating system, 18.3g (0.15mol) of trimethylacetyl chloride was added dropwise to the system, the reaction was exothermic after the dropwise addition, the internal temperature could rise to 83°C, and the dichloroethane was refluxed. After the dropwise addition, the heating system was turned on to continue to keep warm and reflux. Cool down when no hydrogen chloride gas is released. When the internal temperature drops to 15-20 °C, add 13.3 g (0.1 g) of chlorosulfonic acid dropwise. During the dropwise addition, use ice water to control the internal temperature to not exceed 25 °C. The reaction was incubated at -20°C for 1 hour. Add 20% aqueous sodium hydroxide solution and adjust the pH to about 8. At this time, the internal temperature can reach 50-60°C due to acid-base neutralization and exotherm, separate the organic la...
Embodiment 2
[0025] Add 10.5 g (0.1 mol) of 99% 4-methyl-3-thiosemicarbazide (I) and 30 mL of 1,2-dichloroethane to a 100 mL reaction flask, heat it to 80°C, turn off the heating system, 18.3g (0.15mol) of trimethylacetyl chloride was added dropwise to the system, the reaction was exothermic after the dropwise addition, the internal temperature could rise to 83°C, and the dichloroethane was refluxed. After the dropwise addition, the heating system was turned on to continue to keep warm and reflux. When no hydrogen chloride gas is released, 10.2 g (0.1 g) of acetic anhydride was added dropwise under reflux. After the dropwise addition was completed, the reaction was kept under reflux for 1 hour, and the dichloroethane and by-produced acetic acid were recovered under reduced pressure, and 20 mL of dichloroethane was added. Using 20% aqueous sodium hydroxide solution, adjust the pH to about 8, keep the internal temperature at 50-60 ° C, separate the organic layer, and recover dichloroethane ...
Embodiment 3
[0027] Add 10.5 g (0.1 mol) of 99% 4-methyl-3-thiosemicarbazide (I) and 30 mL of 1,2-dichloroethane to a 100 mL reaction flask, heat it to 80°C, turn off the heating system, 18.3g (0.15mol) of trimethylacetyl chloride was added dropwise to the system, the reaction was exothermic after the dropwise addition, the internal temperature could rise to 83°C, and the dichloroethane was refluxed. After the dropwise addition, the heating system was turned on to continue to keep warm and reflux. When no hydrogen chloride gas was released, 7.9 g (0.1 g) of acetyl chloride was added dropwise under reflux. After the dropwise addition was completed, the reaction was kept under reflux for 1 hour, and the dichloroethane and by-product acetic acid were recovered under reduced pressure, and then 20 mL of dichloroethane was added. Using 20% aqueous sodium hydroxide solution, adjust the pH to about 18, keep the internal temperature at 50-60 ° C, separate the organic layer, and recycle dichloroeth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com