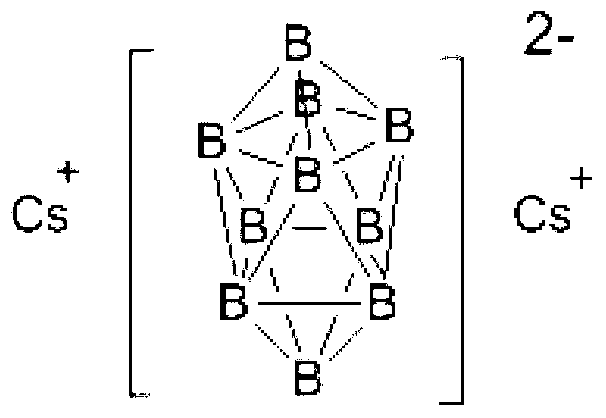
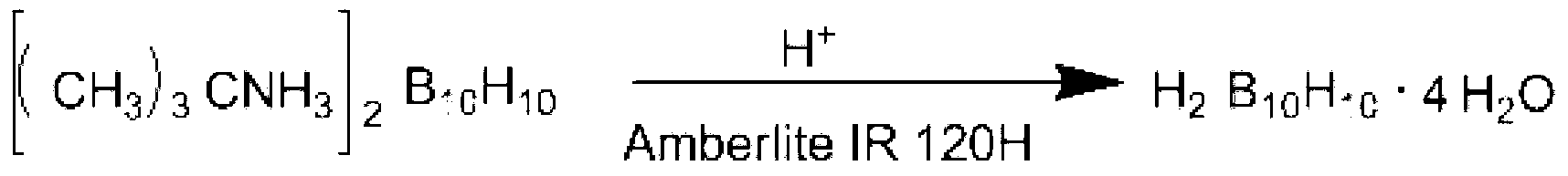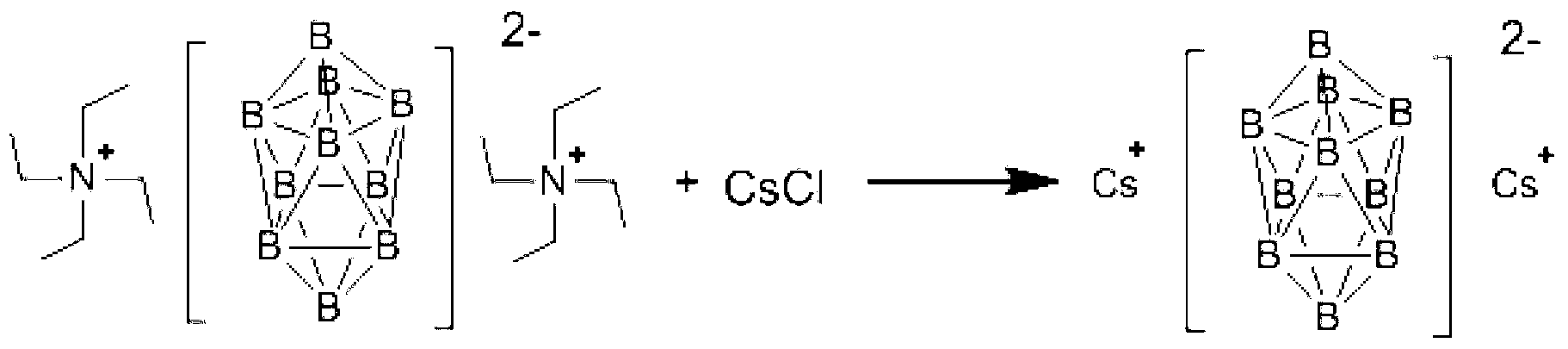Preparation method of caesium decahydrodecaborate
A technology of decahydrodecaboric acid and ditetraethylammonium decahydrodecaborate, which is applied in the field of cesium decahydrodecaborate, can solve the problems of long route and many reaction steps, and achieve high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Step 1: Add 7.6g (20mmol) ditetraethylammonium decahydrodecaborate and 180mL methanol into a reaction flask with a reflux and stirring device, stir, and heat to 60°C to dissolve ditetraethylammonium decahydrodecaborate ;
[0032] Step 2, add 8.0g (48mmol) cesium chloride and 200mL methanol into the reaction flask with condenser tube, stirrer thermometer and feeding valve, heat up to 60°C under stirring to dissolve cesium chloride, keep the temperature, and Add the ditetraethylammonium decahydrodecaborate methanol solution prepared in Step 1 into the reaction flask under strong stirring. After adding the materials, continue to stir and react at 60°C for 2 hours, cool to room temperature, and filter the reaction mixture , washed the filter cake with methanol, and dried the obtained solid to obtain 7.5 g of cesium decahydrodecaborate, with a yield of 98.7%.
[0033] Structure Identification:
[0034] Elemental analysis (B 10 h 10 Cs 2 ):
[0035] Calculated value (%)...
Embodiment 2
[0041] Step 1: Add 7.6g (20mmol) ditetraethylammonium decahydrodecaborate and 150mL methanol into a reaction flask with a reflux and stirring device, stir, and heat to 65°C to dissolve the material;
[0042] Step 2: Add 6.7g (40.2mmol) cesium chloride and 180mL methanol into a reaction flask with a condenser tube, a stirrer thermometer and a feed valve, heat up to 65°C under stirring to dissolve the material, maintain this temperature, and Add the methanol solution of ditetraethylammonium decahydrodecaborate prepared in Step 1 into the reaction flask under stirring. After the addition, continue stirring and reacting for 1 h at 65°C, cool to room temperature, and filter the reaction mixture. The filter cake was washed with methanol, and the obtained solid was dried to obtain 7.3 g of cesium decahydrodecaborate with a yield of 96.1%.
[0043] The structure identification result of the product obtained in this example is the same as that in Example 1.
Embodiment 3
[0045] Step 1: Add 7.6g (20mmol) bistetraethylammonium decahydrodecaborate and 250mL methanol into a reaction flask with a reflux and stirring device, stir, and heat to 40°C to dissolve the material;
[0046] Step 2: Add 16.6g (99.6mmol) of cesium chloride and 976mL of methanol into the reaction flask, heat to 40°C with stirring to dissolve; maintain the temperature, and under strong stirring, decahydrodeca prepared in step 1 Add the methanol solution of ditetraethylammonium borate into the reaction flask, continue to stir and react for 6 hours at a temperature of 40°C, filter, wash the filter cake with methanol, and dry to obtain 7.4 g of cesium decahydrodecaborate, with a yield of 97.4%.
[0047] The structure identification result of the product obtained in this example is the same as that in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com