Target protein for improving spermatogensis
A technology of target protein and spermatogenesis, applied in the field of target protein for improving spermatogenesis, can solve the problems of decreased fertility, decreased sperm count and decreased sperm motility in male mice
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
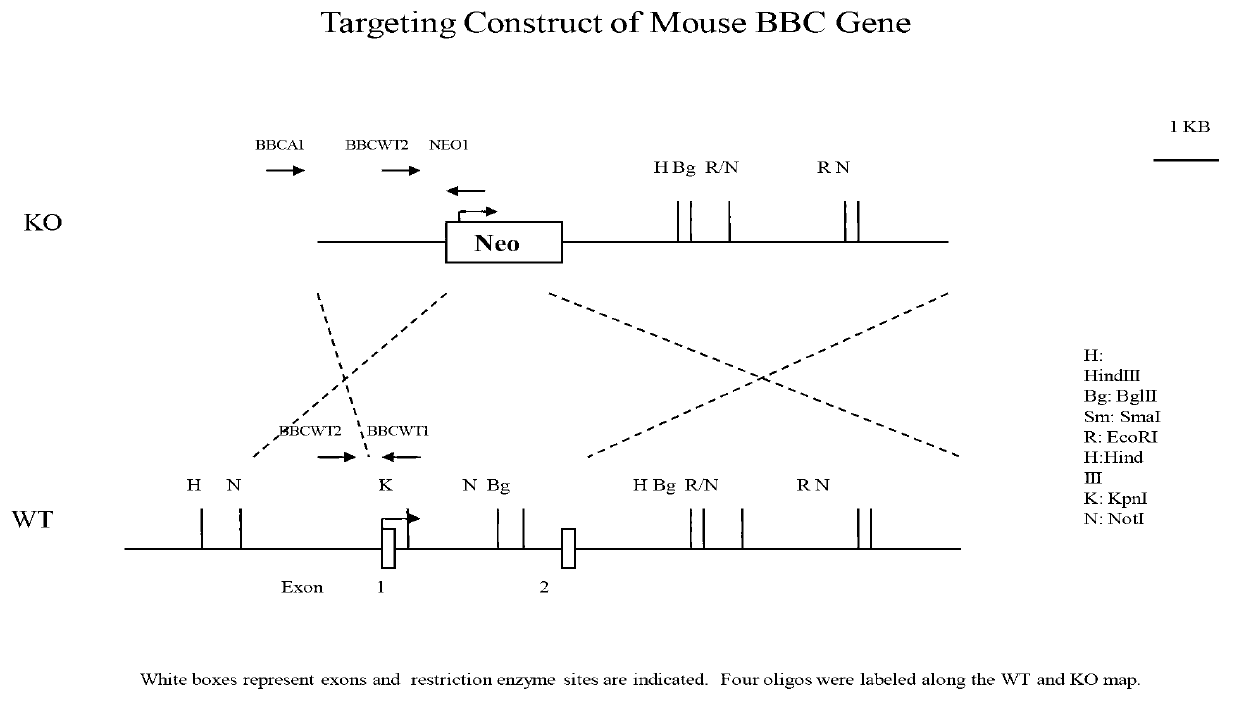
[0532] Example 1, βB 2 Establishment and Identification of Crystallin Knockout Mice Materials and Methods
[0533] 1. Experimental animals
[0534] The animals used in the experiments were mice of C57BL / C strain.
[0535] 2. Experimental materials
[0536] (1) Main reagents and instrument consumables
[0537]
[0538]
[0539] (2) Preparation of main solutions and reagents
[0540] 1. Electrophoresis buffer TBE
[0541] Weigh 2.42g of Tris-Base, 2.75g of boric acid, 1ml of 0.5M EDTA, add double distilled water to make up to 500ml.
[0542] 2. Genome extraction kit related solutions
[0543] Prepare your own absolute ethanol. In the DNA extraction kit, buffer GD and rinse solution PW are prepared by adding absolute ethanol as required before use.
[0544] 3. 0.7~1% agarose gel solution
[0545] Weigh 0.5-0.6 g of agarose, put it into an Erlenmeyer flask, add 80-90 ml of TBE electrophoresis buffer, and dissolve to form an agarose gel solution with a concentration o...
Embodiment 2
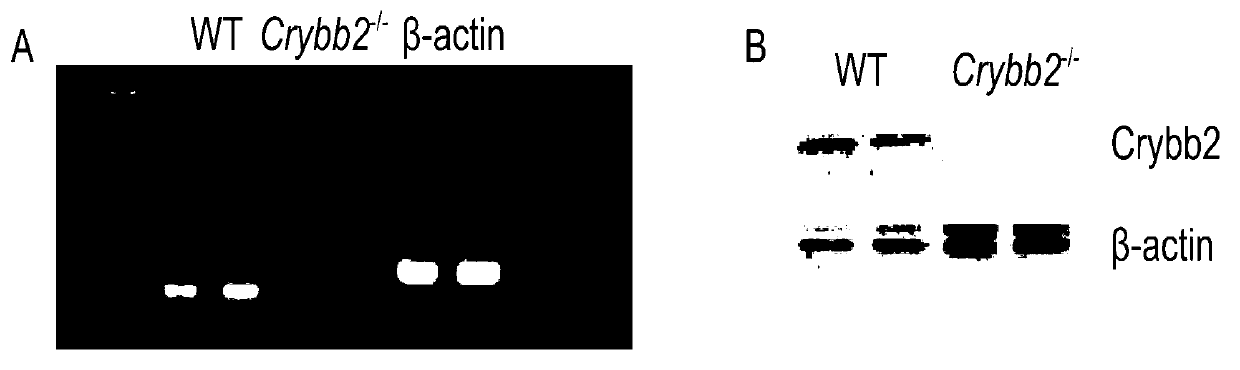
[0600] Example 2, βB 2 Effects of Crystallin Gene Knockout on the Reproductive Function of Male MiceMaterials and Methods
[0601] 1. Experimental materials
[0602] (1) Main reagents
[0603]
[0604]
[0605] (2) Main instruments and consumables
[0606]
[0607] (3) Preparation of main solutions and reagents
[0608] 1. Preparation of related reagents for Western blotting
[0609] (1) 4× protein loading buffer: 5ml of 1M Tris-HCl (pH6.8), 0.8g of SDS, 2ml of glycerol, 0.0012g of bromophenol blue, 0.4ml of β-mercaptoethanol, and dilute to 10ml with deionized water.
[0610] (2) 1.5M Tris-HCl (pH8.8): Weigh 18.671g of Tris, dissolve in 100ml of distilled water, and adjust the pH to 8.8 with concentrated hydrochloric acid.
[0611] (3) 1M Tris-HCl (pH6.8): Weigh 12.114g of Tris, dissolve in 100ml of distilled water, and adjust the pH to 6.8 with concentrated hydrochloric acid.
[0612] (4) 10% ammonium persulfate (abbreviation: AP): 1g of ammonium persulfate pow...
Embodiment 3
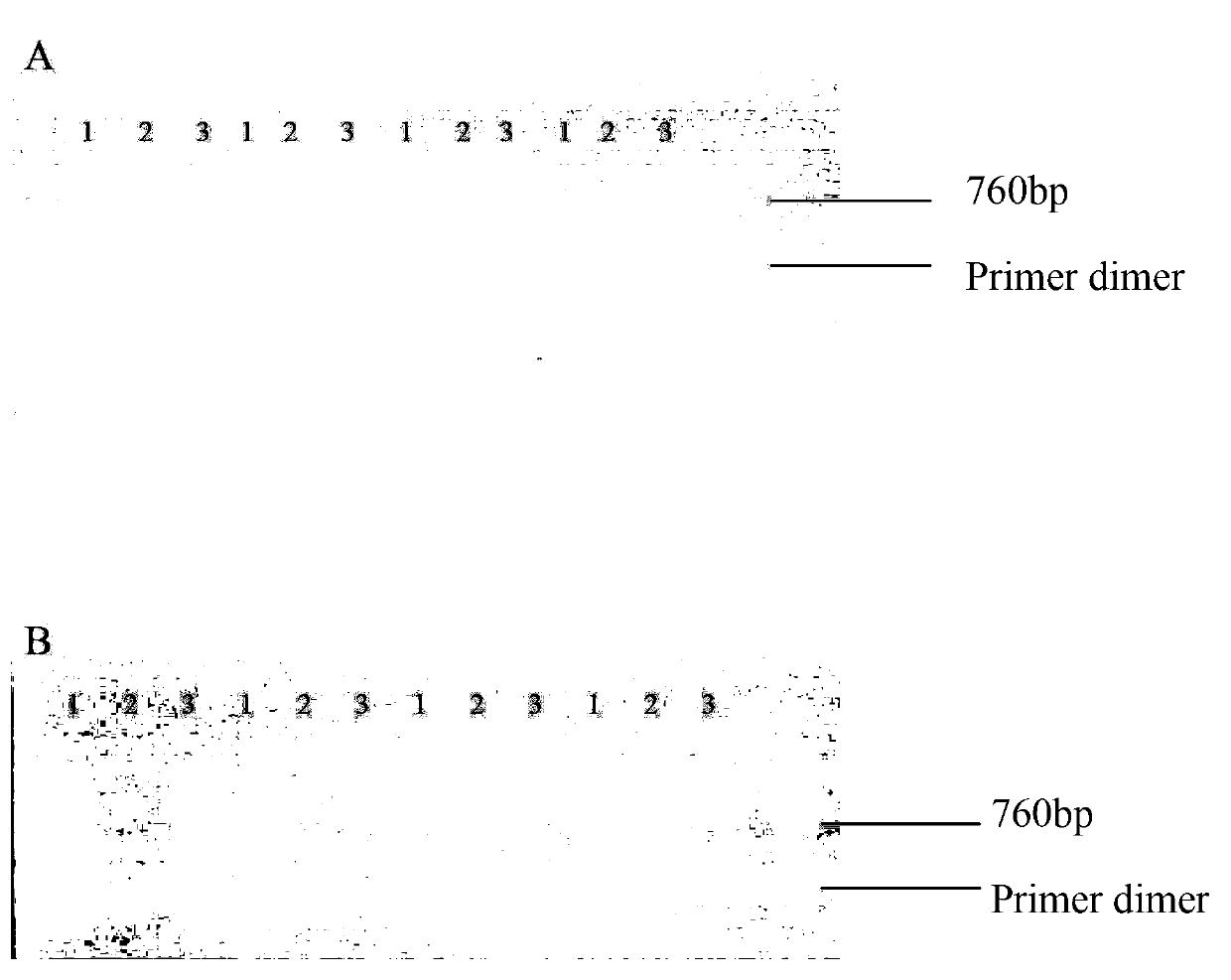
[0749] Example 3, βB2 Crystallin Gene Knockout Causes Mechanism of Reduction of Male Mice Reproductive Function Materials and Methods
[0750] 1. Experimental materials
[0751] (1) Main reagents and instrument consumables
[0752]
[0753] (2) Preparation of main solutions and reagents
[0754] 1. Liquid preparation related to TUNEL detection
[0755] TUNEL detection blocking solution: 3% hydrogen peroxide dissolved in methanol, with 30% H 2 o 2 And 80% methanol solution preparation.
[0756] TUNEL detection permeation solution: 0.1% TritionX-100 dissolved in 0.1% sodium citrate.
[0757] TUNEL detection DNase reaction solution: 100~3000DNase, 40mM Tris-HCL PH7.9, 10mM NaCL, 6mM MgCL2, 10mM CaCL 2 .
[0758] TdT enzyme reaction solution: 45ul equilibration buffer (Equilibration Buffer) + 1ul Biotin-11-dUTP + 4ul TdT Enzyme mixed.
[0759] Streptavidin-HRP working solution: mix 0.5ul Streptavidin-HRP+99.5ul PBS.
[0760] 2. BrdU dissolution
[0761] Dissolve BrdU ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com