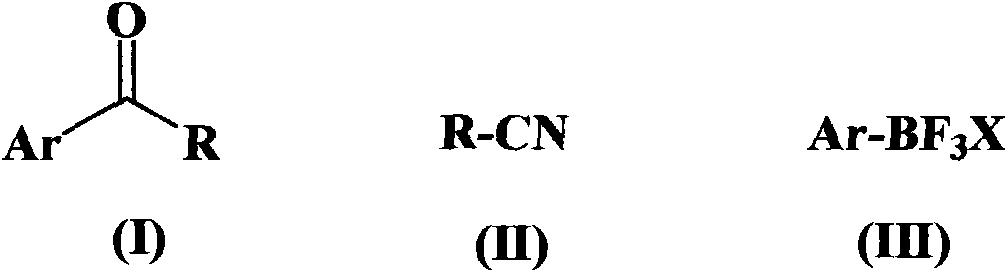
Alkyl and aryl ketone compound preparation method
A technology for alkyl aryl ketones and compounds, which is applied in the field of organic chemical synthesis, can solve the problems of expensive catalyst and low yield, and achieves the effects of simple operation, high yield and increased reaction activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1: the synthesis of methyl phenyl ketone
[0062]
[0063] In a reaction vessel equipped with a stirrer, a thermometer, and a feeding port, add 25ml of tetrahydrofuran, 10mmol of acetonitrile, 10mmol of potassium phenyltrifluoroborate, 0.2mmol of palladium trifluoroacetate, 0.2mmol of 2,2'-bipyridine and 50mmol of trifluoroacetic acid , replaced by nitrogen three times, and then stirred and reacted at 60° C. for 15 hours under the protection of continuous feeding of nitrogen. After the reaction was completed, it was left to cool down to room temperature; the organic layer was separated and extracted with saturated sodium bicarbonate and saturated saline, and evaporated to dryness to obtain the target product with a yield of 98% and a purity of 98.2% (HPLC).
[0064] NMR: 1 H NMR (CDCl 3 , 500MHz) δ7.96(d, J=7.1Hz, 2H), 7.57(t, J=7.4Hz, 1H), 7.47(t, J=7.4Hz, 2H), 2.61(s, 3H);
[0065] 13 C NMR (CDCl 3 , 125MHz) δ198.2, 137.2, 133.1, 128.6, 128.3, 26.6....
Embodiment 2
[0066] Embodiment 2: the synthesis of methyl phenyl ketone
[0067]
[0068] In a reaction vessel equipped with a stirrer, a thermometer, and a feeding port, add 20ml of toluene, 10mmol of acetonitrile, 20mmol of sodium phenyltrifluoroborate, 0.5mmol of palladium trifluoroacetate, and 1mmol of 3,3'-dimethyl-2,2' -Bipyridine and 100 mmol trifluoroacetic acid, replaced with nitrogen three times, and then stirred and reacted at 80° C. for 20 hours under the protection of continuous feeding of nitrogen. After the reaction was completed, it was left to cool down to room temperature; the organic layer was separated and extracted with saturated sodium bicarbonate and saturated saline, and evaporated to dryness to obtain the target product with a yield of 91% and a purity of 99.4% (HPLC).
[0069] NMR: 1 H NMR (CDCl 3 , 500MHz) δ7.96(d, J=7.1Hz, 2H), 7.57(t, J=7.4Hz, 1H), 7.47(t, J=7.4Hz, 2H), 2.61(s, 3H);
[0070] 13 C NMR (CDCl 3 , 125MHz) δ198.2, 137.2, 133.1, 128.6, 128.3,...
Embodiment 3
[0071] Embodiment 3: the synthesis of cuminone
[0072]
[0073] In a reaction vessel equipped with a stirrer, a thermometer, and a feeding port, add 35 ml of dichloromethane, 15 mmol of isobutyronitrile, 45 mmol of potassium phenyltrifluoroborate, 1.5 mmol of palladium trifluoroacetate, 3 mmol of 4,4'-bipyridine and 120 mmol of Trifluoroacetic acid was replaced with nitrogen three times, and then stirred and reacted at 100° C. for 24 hours under the protection of continuous feeding of nitrogen. After the reaction was completed, it was left to cool down to room temperature; the organic layer was separated and extracted with saturated sodium bicarbonate and saturated brine respectively, and the target product was obtained after rotary evaporation to dryness, with a yield of 94% and a purity of 98.9% (HPLC).
[0074] NMR: 1 H NMR (CDCl 3 , 500MHz) δ7.95(d, J=7.7Hz, 2H), 7.55(t, J=7.3Hz, 1H), 7.46(t, J=7.7Hz, 2H), 3.60-3.56(m, 1H), 1.22(d, J=6.9Hz, 6H);
[0075] 13 C NMR ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com