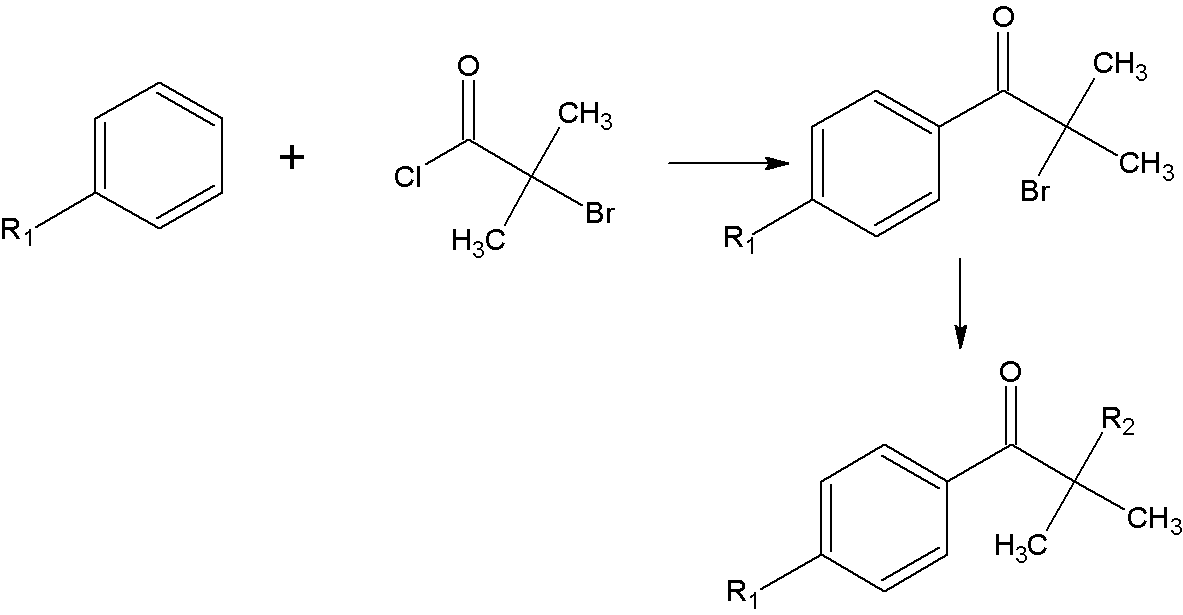
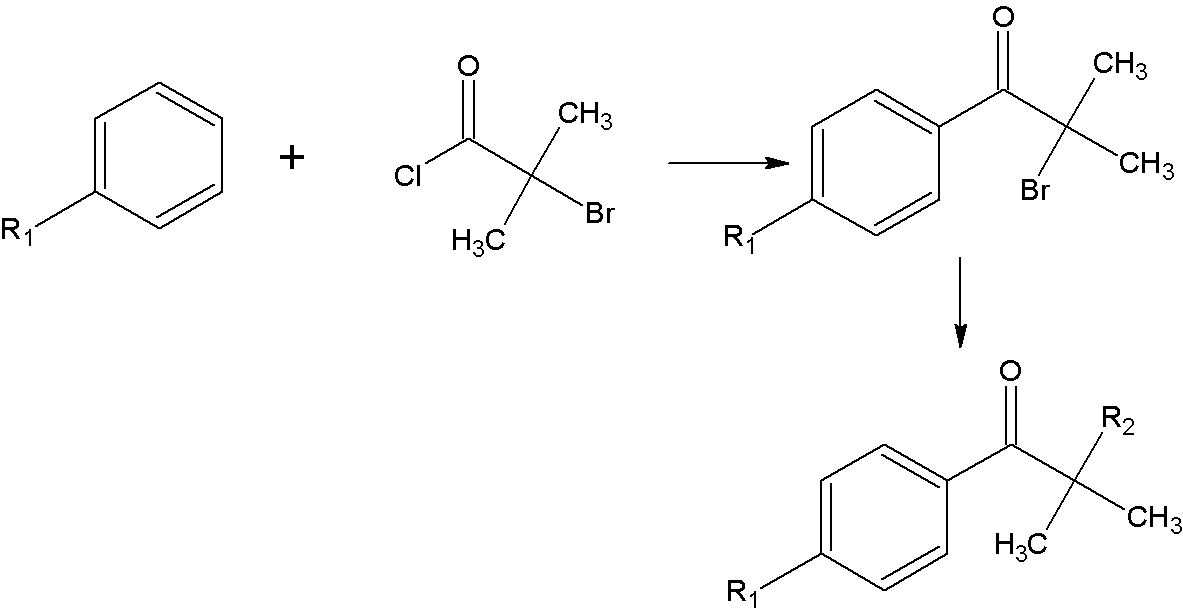
Synthetic method of alpha-amino aromatic ketone compound
A technology of aminoaromatic ketone and synthesis method, which is applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., can solve problems such as difficult industrial production, complicated operation steps, and many reaction steps, and achieve good industrialization prospects, The effect of high yield and purity and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The synthesis of embodiment 1,2-methyl-2-(4-morpholinyl)-1-phenylacetone
[0036] Add 300ml of benzene and 80g (0.6mol) of aluminum trichloride to a four-neck flask equipped with a thermometer, a drying tube, an electromagnetic stirrer, and a constant pressure dropping funnel. After stirring evenly, add 93g ( 0.5mol) α-bromoisobutyryl chloride, react at room temperature for 9h after the dropwise addition is completed. After the reaction, extract with 1000ml 10% hydrochloric acid aqueous solution to obtain the aqueous phase, then extract with 2×100ml benzene to separate the organic phase. The organic phase was adjusted to pH=7 by washing with 2×100ml saturated sodium bicarbonate solution, and the obtained organic phase was distilled under reduced pressure to -0.096mpa to obtain the intermediate product 2-methyl-2-bromo-1-propiophenone.
[0037] Add 300ml of 60-90℃ petroleum ether, 104g (1.2mol) of morpholine, and 6.7g (0.05mol) of aluminum trichloride into a four-neck f...
Embodiment 2
[0038] Embodiment 2, the synthesis of 2-methyl-2-(diethylamino)-1-phenylacetone
[0039] The synthetic method of the intermediate product 2-methyl-2-bromo-1-propiophenone is the same as in Example 1.
[0040] Add 0.2mol 2-methyl-2-bromo-1-propiophenone, 300ml toluene, 2.7g (0.02mol) trichloride aluminum. 60ml (0.6mol) of diethylamine was added dropwise with stirring. After the dropwise addition, react at 55°C for 16h. After the reaction, toluene and diethylamine were distilled off, then 10% hydrochloric acid aqueous solution was added dropwise to adjust the pH of the reaction solution to 2, and 100ml of toluene was added to stir and stand to separate the water phase. Adjust the pH of the aqueous phase to 10 with saturated sodium carbonate solution. The organic phase was separated by extraction with 2×100ml chloroform, and the crude product was obtained by distillation under reduced pressure to -0.096mpa. Recrystallize with the mixed solvent of ethanol and water subsequent...
Embodiment 3
[0041] Embodiment 3, the synthesis of 2-methyl-2-piperidinyl-1-phenylacetone
[0042] The synthetic method of the intermediate product 2-methyl-2-bromo-1-propiophenone is the same as in Example 1.
[0043] In a four-necked flask equipped with a thermometer, reflux condenser, mechanical stirring, and dropping funnel, add 0.2mol 2-methyl-2-bromo-1-propiophenone, 400ml benzene, 27g (0.2mol) aluminum trichloride . 30ml (0.3mol) of piperidine was added dropwise with stirring. After the dropwise addition, react at 40°C for 6h. After the reaction, benzene and piperidine were distilled off, then 10% hydrochloric acid aqueous solution was added dropwise to adjust the pH of the reaction solution to 2, and 100ml of benzene was added to stir and stand still to separate the water phase. Use saturated sodium carbonate solution to adjust the pH of the aqueous phase to 10, then extract with 2×100ml chloroform to separate the organic phase, and distill under reduced pressure to -0.096mpa to o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com