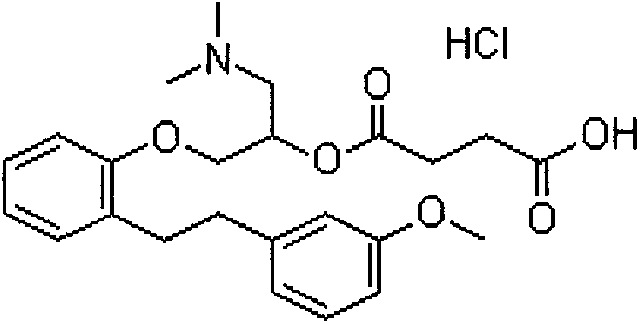
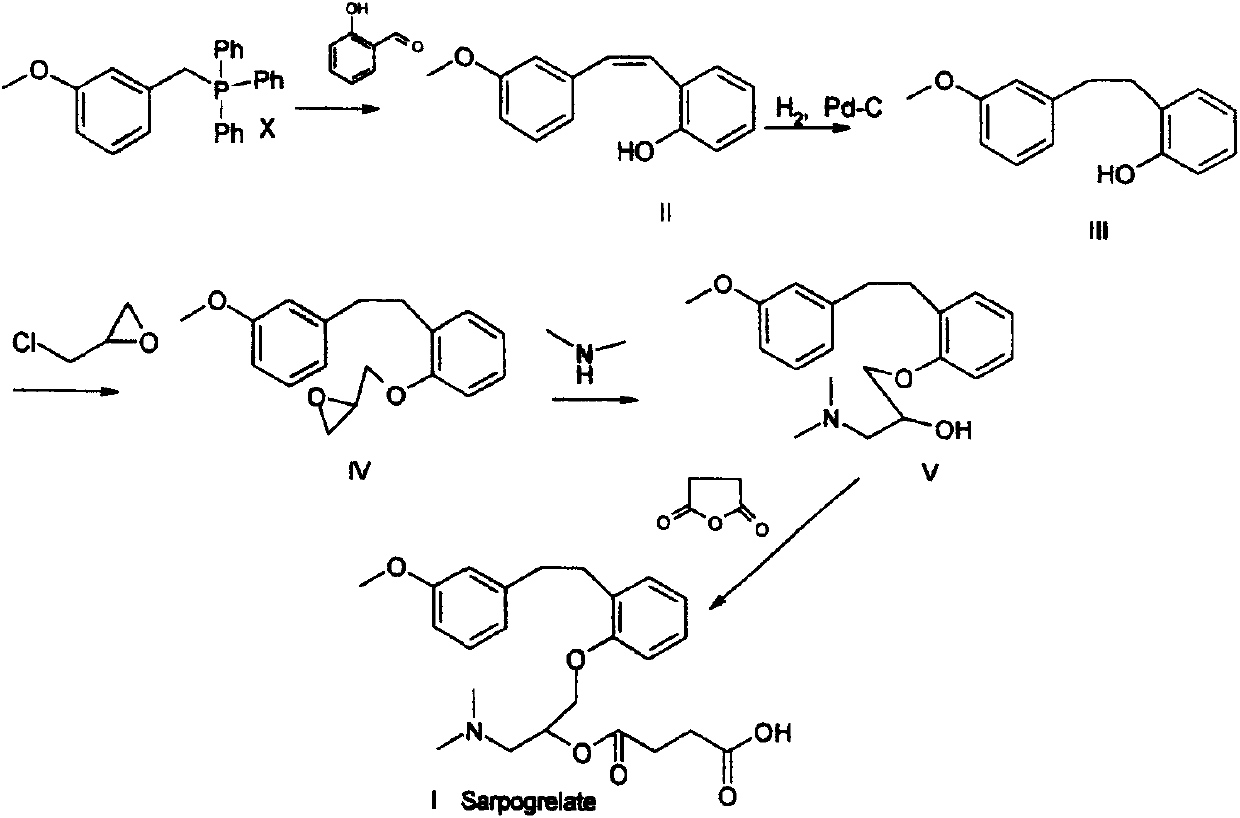
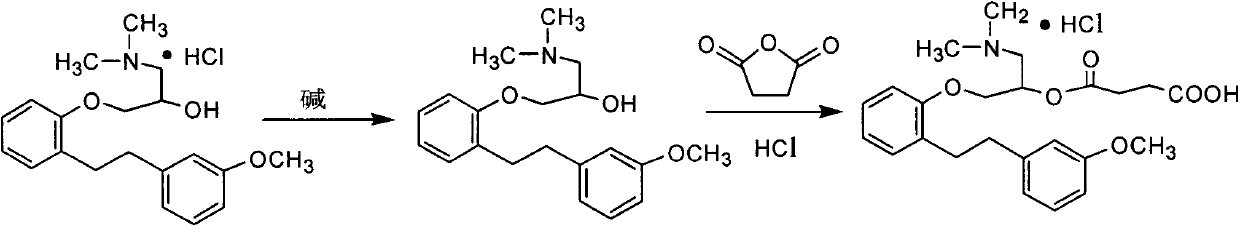
Preparation method of high-purity sarpogrelate hydrochloride
A technology of sagrelate hydrochloride and crystallization temperature, which is applied in the field of preparation of 5-HT2 receptor blocker sarcogrelate hydrochloride, can solve the problems of not mentioning the control of single impurity content and the inability to obtain high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1 Preparation of Crude Sagrelate Hydrochloride
[0020] 1-Dimethylamino-3-[2-[2-(3-methoxyphenyl)ethyl]phenoxy]-2-propanol hydrochloride 13.7g into a 250ml single-neck bottle, and then add water 25ml, stir to dissolve. Adjust the pH value to 9-14 with 20% sodium hydroxide aqueous solution, extract with 30 ml of toluene, and concentrate the organic layer under reduced pressure at 50° C. until there is no leakage of 0 liquid to obtain a brown oil. Then add 30g of tetrahydrofuran, stir to dissolve, add 4.5g of succinic anhydride, stir and heat to reflux, reflux for 1 to 4 hours, then concentrate the reaction solution at 40°C under reduced pressure to dryness; add 25g of ethyl acetate to the residue and stir After dissolving, add dropwise saturated hydrogen chloride ethyl acetate solution to adjust the PH value to below 1, and stir for 50-60 min. Centrifugal suction filtration, a crude wet product of sargresil hydrochloride was obtained. Dry at 45~55℃ under reduced ...
Embodiment 2
[0021] Example 2 Purification of Crude Sagrexate Hydrochloride
[0022] Take 5g of crude sargrelate hydrochloride, add 20ml of methyl ethyl ketone, stir and warm to dissolve, reflux for 20-30 minutes, cool to 25-35°C, keep stirring for 40-60 minutes, filter, and rinse the filter cake with a small amount of methyl ethyl ketone to obtain a white loose The solid was dried under reduced pressure at 55-65°C for 24 hours to obtain 4.6 g of sargrelate hydrochloride, with a yield of 92%, HPLC purity of 99.9%, and a maximum single impurity content of 0.04%.
Embodiment 3
[0023] Example 3 Purification of Crude Sagrelate Hydrochloride
[0024] Take 5g of crude sargrelate hydrochloride, add 30ml of methyl ethyl ketone, stir and warm to dissolve, reflux for 20-30 minutes, cool to 25-35°C, keep stirring for 40-60 minutes, filter, and rinse the filter cake with a small amount of methyl ethyl ketone to obtain a white loose The solid was dried under reduced pressure at 55-65°C for 24 hours to obtain sargrelate hydrochloride 4.55, yield 91%, HPLC purity 99.7%, and maximum single impurity content 0.05%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com