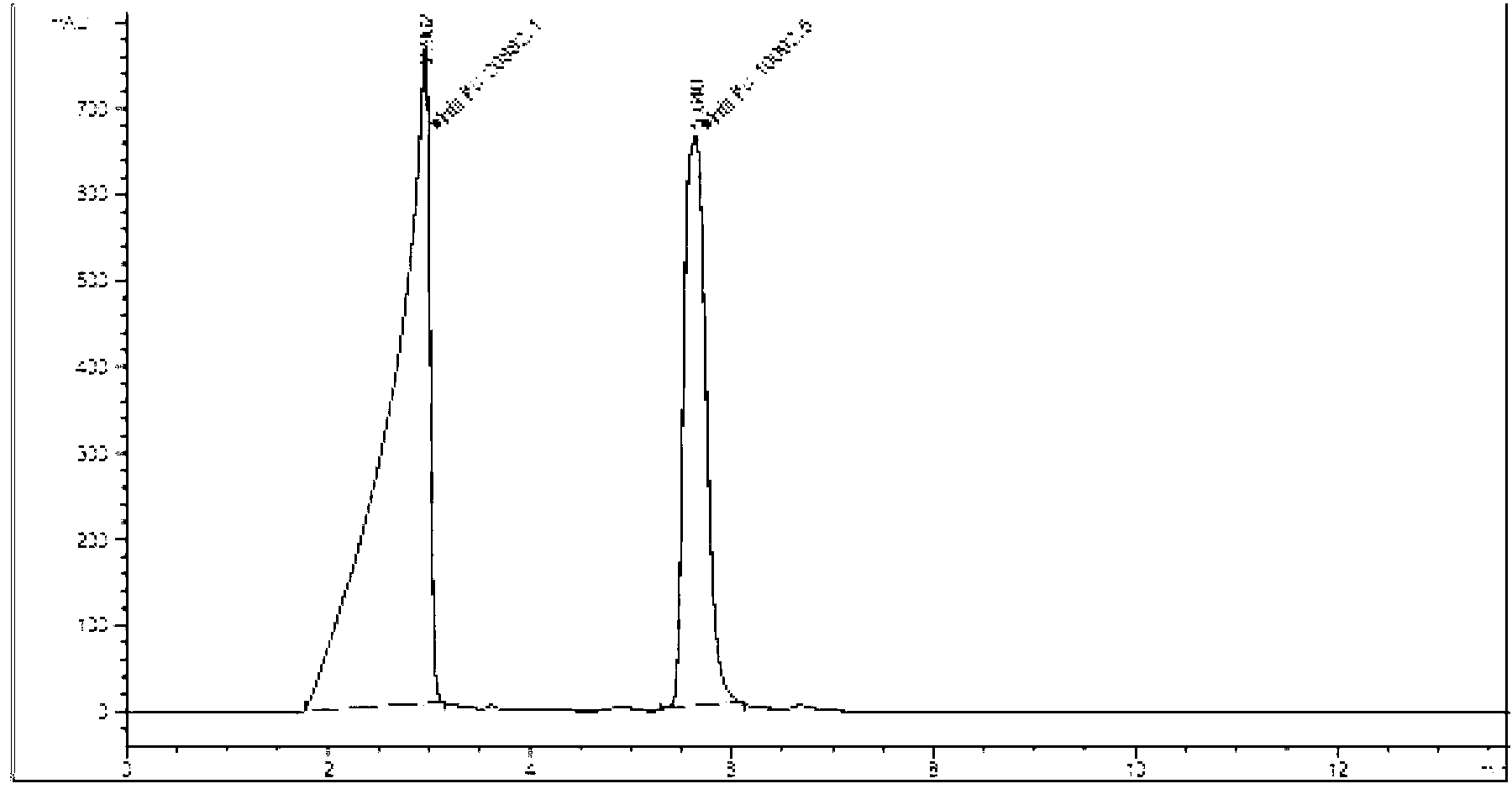
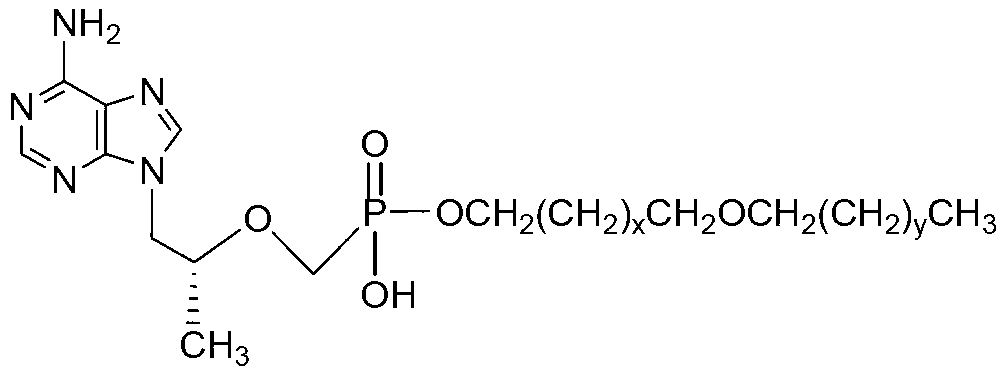
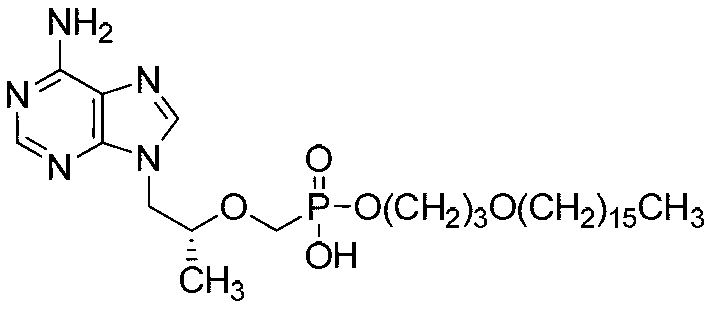
Tenofovir disoproxil compounds, and preparation method and application thereof in anti-virus aspects
A technology of tenofovir disoproxil and compound, applied in the field of nucleoside-like compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Embodiment 1: the preparation of 3-hexadecyloxy-1-propanol (L114)
[0073]
[0074] In a 250ml three-neck round bottom flask, add 1,3-propanediol (9.13g, 0.12mol), potassium tert-butoxide (6.8g, 0.06mol) and tert-amyl alcohol (50ml) in sequence, and slowly add A mixture of hexadecane bromide (12.17g, 12.2ml, 0.04mol) and tetrahydrofuran (50ml) was added dropwise over 3 hours. After refluxing and stirring for 50 hours, cool to room temperature, pour the reaction solution into 50ml of water, stir, acidify with 10% hydrochloric acid to pH=7, add n-hexane (100ml), separate the organic phase, and extract the aqueous phase with n-hexane , the organic phases were combined, dried and concentrated, and then recrystallized with n-pentane to obtain 3-hexadecyloxy-1-propanol (L114) (7.8 g, 0.026 mol), yield: 65%.
[0075] 1 H NMR (400MHz, CDCl 3 )δ,(ppm):0.88(3H,t,CH 3 ),1.14-1.37(26H,m,13×CH 2 ),1.48-1.65(2H,m,CH 2 ), 1.71-1.94 (2H, m, CH 2 ),2.38-2.53(1H,br,OH),3.43(2H,...
Embodiment 2
[0076] Embodiment 2: the preparation of 2-octadecyloxyethanol (L016)
[0077]
[0078] Synthesize 2-octadecyloxyethanol (L016) with the method similar to embodiment 1
[0079] 1 H NMR (400MHz, CDCl 3 )δ,(ppm):0.88(3H,t,CH 3 ),1.06-1.49(30H,m,15×CH 2 ), 1.53-1.654 (2H, m, CH 2 ), 1.90-2.10 (1H, br, OH), 3.47 (2H, t, OCH 2 ),3.53(2H,t,OCH 2 ),3.73(2H,t,OCH 2 ). ESI-MS:[M+H] + 315.3, [M+Na] + 337.3.
Embodiment 3
[0080] Example 3: Preparation of 6-dodecyloxy-1-hexanol (L410)
[0081]
[0082] 6-dodecyloxy-1-hexanol (L410) was synthesized in a similar manner to Example 1. 1 H NMR (400MHz, CDCl 3 )δ,(ppm):0.88(3H,t,CH 3 ),1.14-1.34(18H,m,9×CH 2 ),1.35-1.42(4H,m,2×CH 2 ),1.48-1.64(6H,m,3×CH 2 ),1.93-2.01(1H,br,OH),3.28-3.48(4H,m,2×OCH 2 ),3.62(2H,t,OCH 2 ). ESI-MS:[M+H] + 287.3, [M+Na] + 309.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com