Drug composition of bortezomib and preparation method thereof
A technology of bortezomib and its composition, which is applied in the field of medicine, can solve the problems of poor resolubility of freeze-dried powder injections, and achieve the effects of accelerating dissolution rate, excellent stability, and increasing clarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Preparation Example 1, Bortezomib freeze-dried powder injection
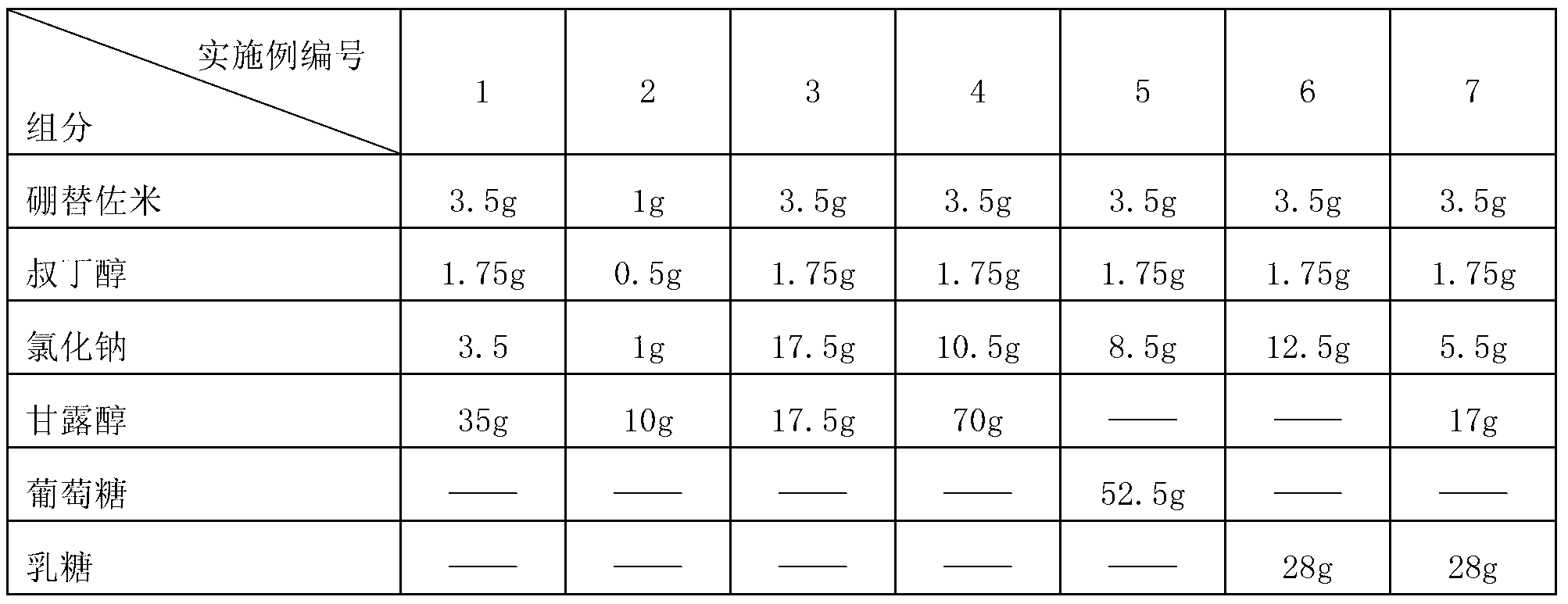
[0047] Prescription:
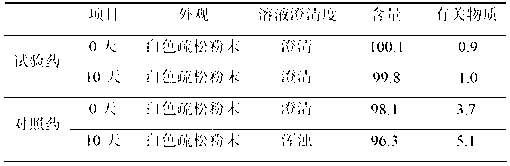
[0048]
[0049]
[0050] Preparation:
[0051] 1) Weigh 3.5g bortezomib, 1.75g tert-butanol, 35g mannitol and 3.5g sodium chloride according to the above prescription;
[0052] 2) Add the prescribed amount of bortezomib into the liquid preparation tank, then add the prescribed amount of tert-butanol, seal the container to obtain a suspension, heat the suspension to 45°C for 5 minutes to completely dissolve the drug, and obtain a solution;
[0053] 3) Add 80% of water for injection to the solution in step 2), stir, then add the prescribed amount of mannitol and sodium chloride, stir the resulting mixture to dissolve completely, cool to room temperature; then add 20% of the prescribed amount of water for injection, stir to fully uniform;
[0054] 4) The prepared solution is sterilized by secondary terminal filtration through a 0.22 μm microporous membrane to obtain a filt...
Embodiment 2
[0059] Formulation example 2, bortezomib freeze-dried powder injection
[0060] Prescription:
[0061]
[0062] Preparation:
[0063] 1) Weigh 3.5g bortezomib, 1.75g tert-butanol, 17.55g mannitol and 17.5g sodium chloride according to the above prescription;
[0064] 2) Add the prescribed amount of bortezomib to the liquid preparation tank, then add the prescribed amount of tert-butanol, seal the container to obtain a suspension, heat the suspension to 40°C for 3 minutes to completely dissolve the drug, and obtain a solution;
[0065] Step 3) and step 4) are the same as the preparation example 1;
[0066] The low-temperature freeze-drying described in step 5) includes the following three stages:
[0067] Pre-freezing stage: lower the temperature of the shelf to -50°C, put the product in quickly, and keep the temperature for 3 hours after the temperature of the product reaches -37°C, and keep the vacuum in the box at 8Pa;
[0068] Primary drying stage: keep the vacuu...
Embodiment 3
[0070] Preparation Example 3, Bortezomib freeze-dried powder injection
[0071] Prescription:
[0072]
[0073] Preparation:
[0074] 1) Weigh 3.5g bortezomib, 1.75g tert-butanol, 70g mannitol and 10.5g sodium chloride according to the above prescription;
[0075] 2) Add the prescribed amount of bortezomib into the liquid preparation tank, then add the prescribed amount of tert-butanol, seal the container to obtain a suspension, heat the suspension to 48°C for 8 minutes to completely dissolve the drug, and obtain a solution;
[0076] Step 3) and step 4) are the same as the preparation example 1;
[0077] The low-temperature freeze-drying described in step 5) includes the following three stages:
[0078] Pre-freezing stage: lower the temperature of the shelf to -50°C, put the product in quickly, and keep the temperature for 5 hours after the temperature of the product reaches -33°C, and keep the vacuum in the box at 12Pa;
[0079] Primary drying stage: keep the vacuu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com