Anticancer compounds and their applications
A compound, cancer technology, applied in the direction of steroids, silicon compounds, active ingredients, antitumor drugs, etc., can solve rare problems and achieve the effect of no toxic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Embodiment 1: the synthesis of lanosterol derivative
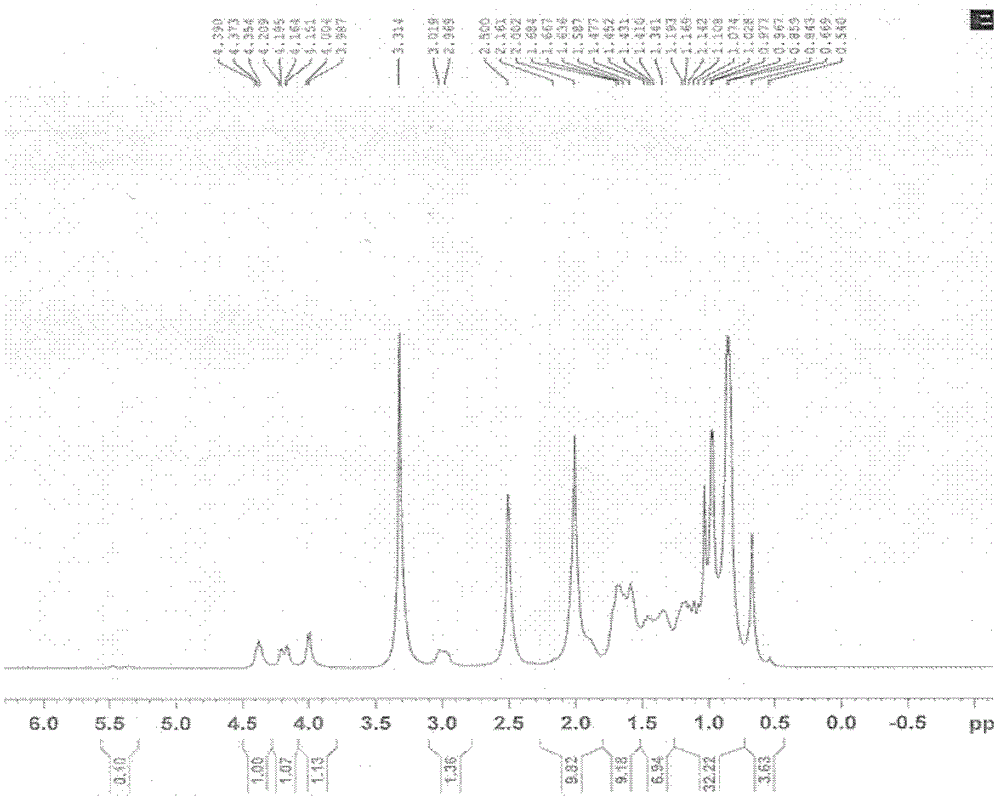
[0085] (1) Dissolve 5 grams of lanosterol in 20 milliliters of pyridine. After complete dissolution, add 2 milliliters of acetic anhydride (about 2 equivalents) dropwise, and react at room temperature for 12 hours to end the reaction. The reaction mixture was concentrated under reduced pressure, and the concentrate was cooled with an ice bath, and a 10% aqueous solution of sodium bicarbonate was slowly added dropwise until no bubbles emerged completely, extracted three times with ethyl acetate, and the organic layers were combined. The organic layer was washed with saturated aqueous sodium chloride solution, dried with anhydrous sodium sulfate, filtered, and the organic layer was spin-dried to obtain 5.5 grams of crude product. After column separation, 4.5 grams of the target product were obtained as a white solid with a yield of 84 %. MS(ESI): m / z569[M+H + ]; the hydrogen spectrum measured by the target product i...
Embodiment 2
[0103] Embodiment 2: the synthesis of lanosterol derivative
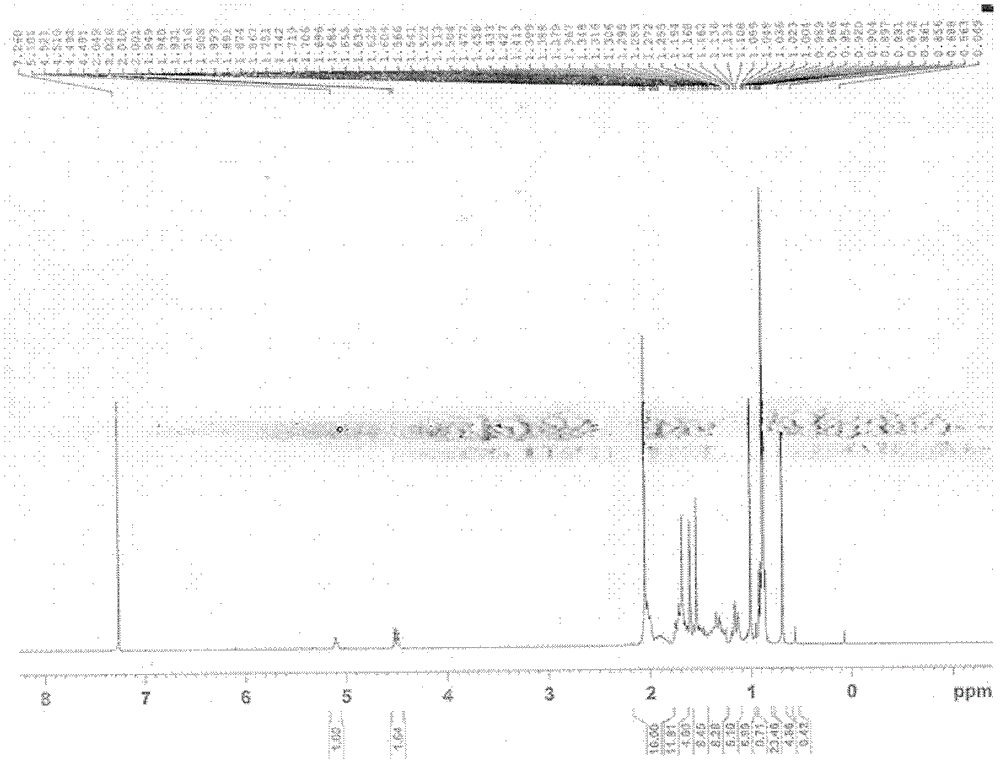
[0104] Dissolve 0.1 g of LD020 in 10 ml of dichloromethane, add 80 mg (about 3 equivalents) of m-chloroperoxybenzoic acid under vigorous stirring, and react at 0°C for 10 hours. Add 0.1 g of calcium hydroxide, stir at room temperature for 1 hour, filter out the solid, wash the solid with 50 ml of dichloromethane, combine the filtrates, concentrate to obtain crude products, and separate on the column to obtain 20 mg of the following LD026-1 respectively The product (yield 18%) and 30 mg of the following product No. LD026-2 (yield 28%) were both white solids. The hydrogen spectrum shows that the hydrogen spectrum of LD026-1 and LD026-2 is the same as that of compound LD020 Figure 1 Sample. Product No. LD026-1 MS(ESI): m / z485[M+H + ], LD026-2 product MS (ESI): m / z 501 [M+H + ]. The reaction equation is as follows:
[0105]
Embodiment 3
[0106] Embodiment 3: the synthesis of lanosterol derivative
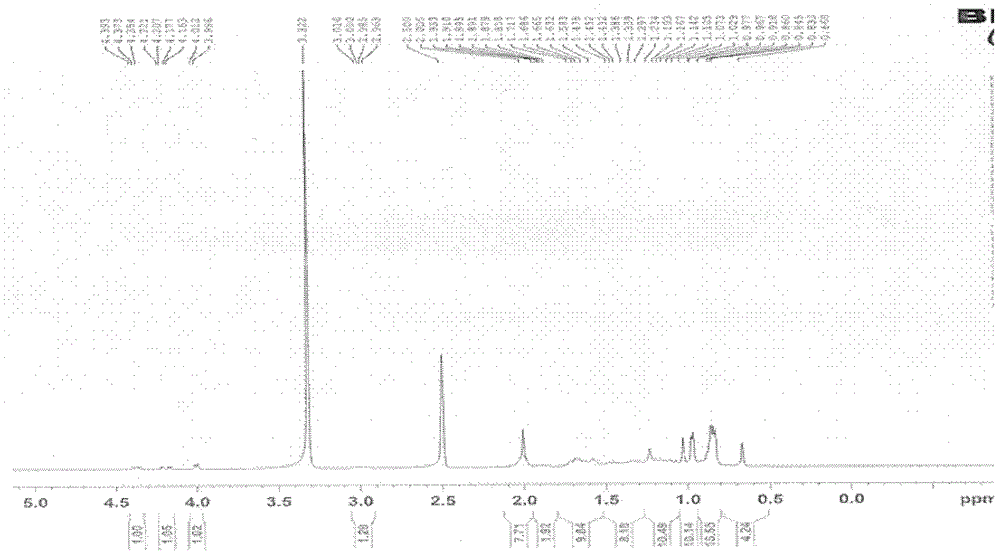
[0107] Dissolve 0.1 g of LD021 in 10 ml of dichloromethane, add 40 mg (about 1.5 equivalents) of m-chloroperoxybenzoic acid under vigorous stirring, and react at 0°C for 10 hours. Add 0.1 g of calcium hydroxide and stir at room temperature for 1 hour. The solid was filtered out, and the solid was washed with 50 ml of dichloromethane. The filtrates were combined and concentrated to obtain a crude product, which was separated on a column to obtain 10 mg of the target product with a yield of 10% as a white solid. Proton spectra showed that LD0027 had the same spectrum as compound LD021. MS(ESI): m / z519[M+H + ]. The reaction equation is as follows:
[0108]
[0109] In order to illustrate the technical scheme of the present invention, the compound number is LD027.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com