Method for preparing xanthene amerantrone derivative via catalysis of acidic ionic liquid
An acidic ionic liquid, xanthene dione technology, applied in chemical recovery, organic chemistry and other directions, can solve the problems of large loss of catalyst usage, harsh reaction conditions, environmental pollution, etc., and achieve less catalyst usage and catalytic efficiency. The effect of reducing and good catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
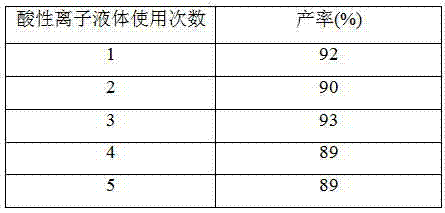
[0018] Add 10mmol of benzaldehyde, 20mmol of 1,3-cyclohexanedione, 15mL of absolute ethanol and 0.5mmol of acidic ionic liquid into 50mL one-necked flasks equipped with stirring bars and reflux condensers. Reflux reaction for 3 hours under vigorous stirring, add ice water to cool, filter, recrystallize the filter residue with 95% ethanol, and dry to obtain pure 9-phenyl-2,3,4,5,6,7-hexahydro-2H-oxa Anthracene-1,8-dione, 92% yield. The acidic ionic liquid catalyst in the filtrate can be recycled after rotary evaporation under reduced pressure to remove water and ethanol, and vacuum drying at 110° C. for 2 h.
Embodiment 2
[0020] Add 10mmol of 4-methoxybenzaldehyde, 20mmol of 1,3-cyclohexanedione, 20mL of absolute ethanol and 0.6mmol of acidic ionic liquid into a 50mL single-necked flask equipped with a stirring bar and a reflux condenser. Reflux for 3.5 hours under vigorous stirring, add ice water to cool, filter, and recrystallize the filter residue with 95% ethanol, dry to obtain pure 9-(4-methoxyphenyl)-2,3,4,5,6,7 - Hexahydro-2H-xanthene-1,8-dione in 95% yield. The acidic ionic liquid catalyst in the filtrate can be recycled after rotary evaporation under reduced pressure to remove water and ethanol, and vacuum drying at 110° C. for 2 h.
Embodiment 3
[0022] Add 10mmol of benzaldehyde, 20mmol of 5,5-dimethyl-1,3-cyclohexanedione, 20mL of absolute ethanol and 0.8mmol of acidic ionic liquid into a 50mL single-necked flask equipped with a stirring bar and a reflux condenser. Under reflux under vigorous stirring for 3 hours, add ice water to cool, filter, recrystallize the filter residue with 95% ethanol, and dry to obtain pure 3,3,6,6-tetramethyl-9-phenyl-2,3,4,5 , 6,7-Hexahydro-2H-xanthene-1,8-dione in 94% yield. The acidic ionic liquid catalyst in the filtrate can be recycled after rotary evaporation to remove water and ethanol, and vacuum drying at 110° C. for 2 h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com