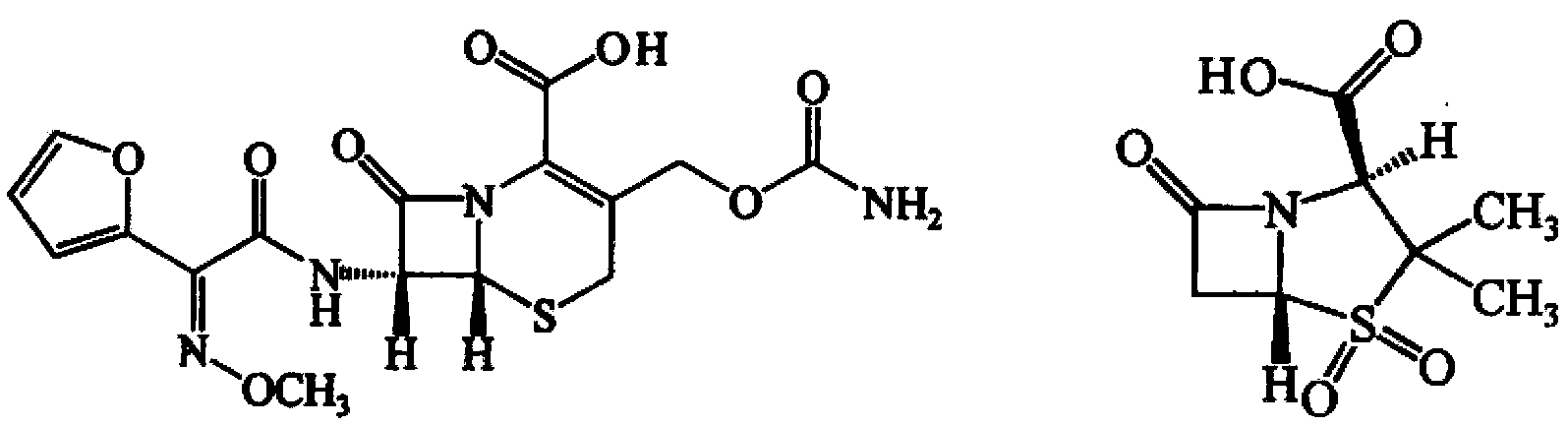
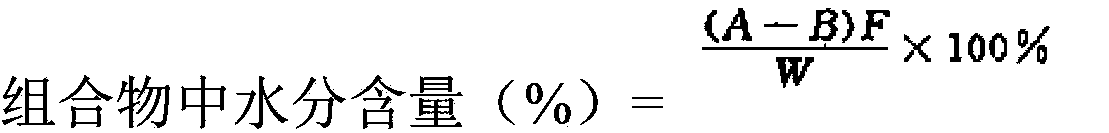Cephalosporin medicinal composition
A composition and drug technology, applied in the direction of drug combination, active ingredients of heterocyclic compounds, anti-infective drugs, etc., can solve the problems of large differences between batches, large differences in loading volume, and high water content of products, and achieve stable quality and proportion. Stable and uniform effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Three batches of compound I and compound II raw materials purchased from different manufacturers were tested according to the standards of compound I and compound II in the second part of the 2010 edition of "Chinese Pharmacopoeia". Meet the relevant requirements of the standard.
[0061] Take 3 batches of compound I and 3 batches of compound II to pulverize and sieve respectively, each batch screened out D 90 Groups I and D of compounds whose particle sizes are 175 μm, respectively 90 Groups of Compound II whose particle size is 175 μm; 1500 g of Compound I (based on the weight of the free acid of Compound I) and 90 Compound II750g (by weight of compound II free acid) taken by each compound II group with the same particle size was mixed uniformly; to obtain D 90 There are 27 groups of pharmaceutical compositions of Compound I and Compound II with particle sizes 175 μm respectively (the weight of Compound I calculated as Compound I free acid and the weight of Compound...
Embodiment 2
[0063] Prepare according to the preparation method of Example 1 to obtain 27 groups of pharmaceutical compositions of Compound I and Compound II after packaging (compound I in the pharmaceutical composition in terms of Compound I free acid and Compound II in terms of Compound II free acid The weight ratio is 3:1), from the 27 groups of pharmaceutical compositions of compound I and compound II after packaging, 9 groups of pharmaceutical compositions with different particle sizes and moisture were screened for use. The particle size and moisture content of each group of pharmaceutical compositions are as follows: Shown in Table 1; where D 90 Table 2 shows the water content of the nine groups of pharmaceutical compositions of Compound I and Compound II with particle sizes ranging from 95 μm to 175 μm.
Embodiment 3
[0065] Prepare according to the preparation method of Example 1 to obtain the pharmaceutical composition of Compound I and Compound II (the weight ratio of Compound I in terms of Compound I free acid and Compound II in terms of Compound II free acid in the pharmaceutical composition is 4:1 ). Screen out 9 groups of pharmaceutical compositions with different particle sizes and moisture from the prepared pharmaceutical compositions of Compound I and Compound II for subsequent use, and the particle size and moisture content of each group of pharmaceutical compositions are as shown in Table 1; wherein D 90 Table 2 shows the water content of the nine groups of pharmaceutical compositions of Compound I and Compound II with particle sizes ranging from 95 μm to 175 μm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com