Cellulose derivative simultaneously containing cyclohexyl carbamate and phenyl carbamate and preparation method of cellulose derivative
A technology of cyclohexyl carbamate and phenyl carbamate, applied in the field of preparation of cellulose derivatives, can solve problems such as poor chiral recognition ability, and achieve the effects of simple operation, mature technology and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
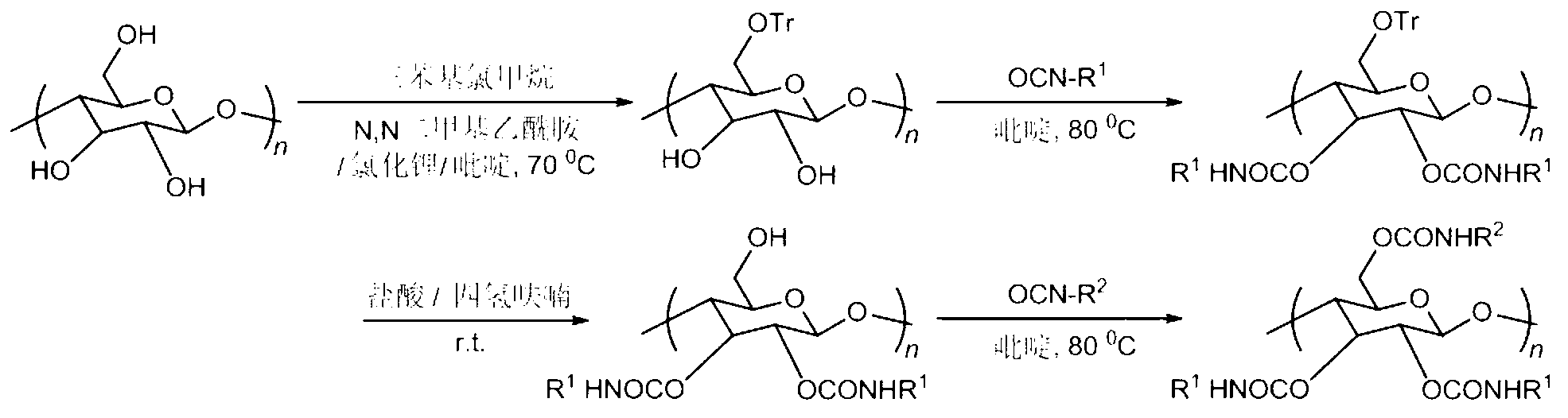
[0029] 1. Take 0.2g of microcrystalline cellulose and vacuum-dry at 80°C for 4h, then stir and reflux in anhydrous N,N-dimethylacetamide for 12h; add 0.4g of lithium chloride after cooling to room temperature; continue to stir for 2h Afterwards, heat up to 70°C again, add anhydrous pyridine, add excess triphenylchloromethane after reflux for 4h, stop the reaction after continuing to stir and reflux for 24h; cool to room temperature, add methanol to settle, filter and wash, and vacuum dry at 60°C To constant weight, the yield was 85%.
[0030] 2. Continue vacuum drying the above intermediate product at 80°C for 4h, then reflux in anhydrous pyridine for 2h, add excess 3,5-dimethylphenylisocyanate, continue to reflux at 80°C for 16h, then stop the reaction , washed well with methanol and dried in vacuo.
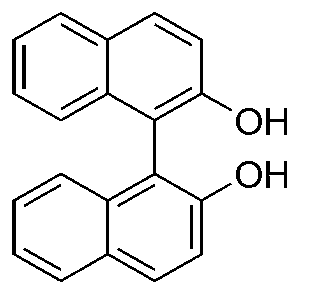
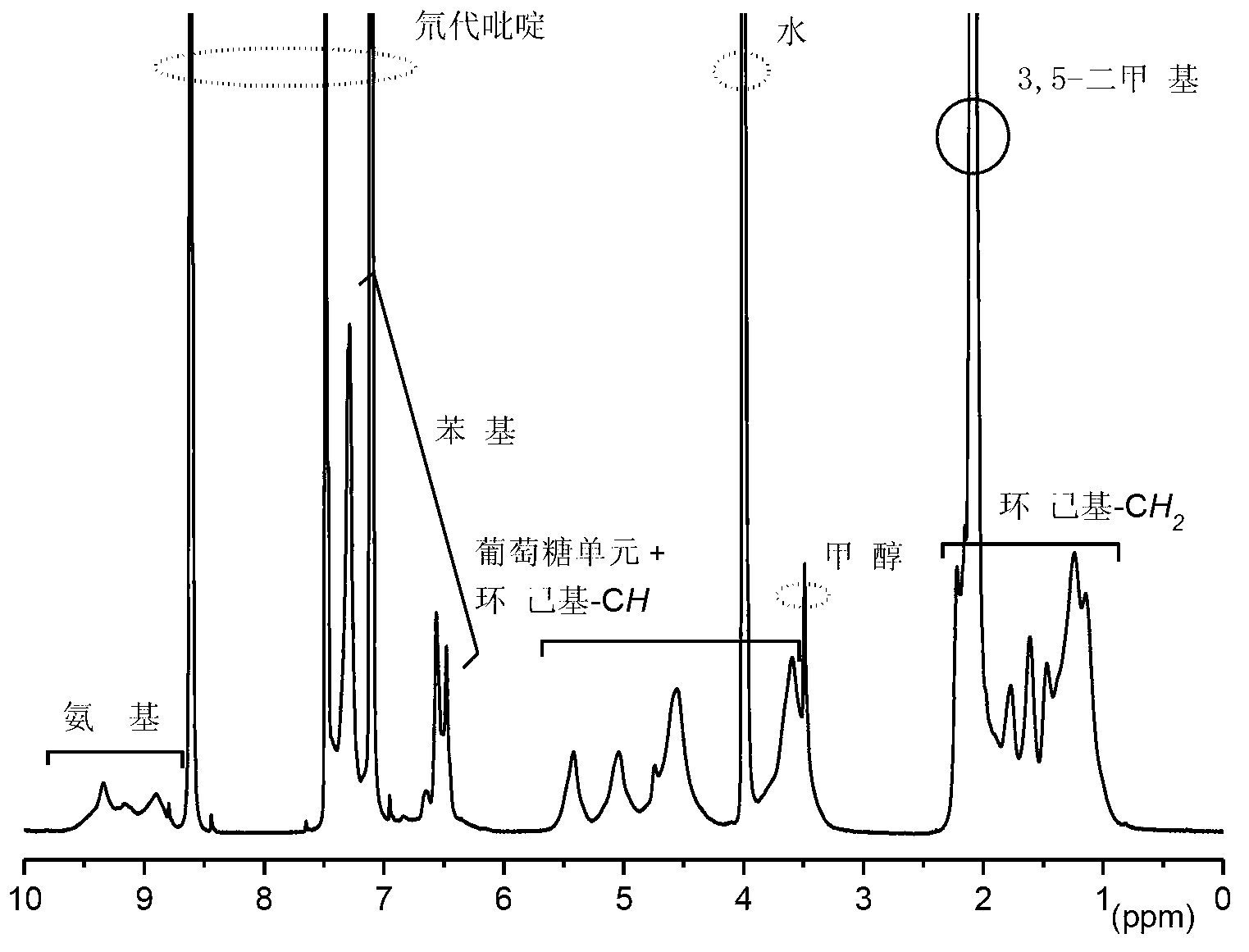
[0031] 3. The intermediate product obtained in the previous step was dissolved in a tetrahydrofuran solution containing a small amount of hydrochloric acid (1.8% by volume of t...
specific Embodiment approach 2
[0035]1. Take 0.2g microcrystalline cellulose and dry it in vacuum at 80°C for 4h, then stir and reflux in anhydrous N,N-dimethylacetamide for 10h; add 0.4g lithium chloride after cooling to room temperature; continue stirring for 4h Afterwards, heat up to 70°C again, add anhydrous pyridine, add excess triphenylchloromethane after reflux for 4h, stop the reaction after continuing to stir and reflux for 24h; cool to room temperature, add methanol to settle, filter and wash, and vacuum dry at 60°C To constant weight, the yield was 87%.
[0036] 2. Continue vacuum drying the above intermediate product at 80°C for 4h, then add excess 3,5-dichlorophenylisocyanate after reflux in anhydrous pyridine for 2h, stop the reaction after continuing to reflux at 80°C for 12h, Wash well with methanol and dry under vacuum.
[0037] 3. Dissolve the intermediate product obtained in the previous step in a tetrahydrofuran solution containing a small amount of hydrochloric acid (2% by volume of te...
specific Embodiment approach 3
[0042] 1. Take 0.3g of microcrystalline cellulose and vacuum-dry at 80°C for 4h, then stir and reflux in anhydrous N,N-dimethylacetamide for 14h; add 0.6g of lithium chloride after cooling to room temperature; continue to stir for 5h Afterwards, heat up to 70°C again, add anhydrous pyridine, add excess triphenylchloromethane after reflux for 4h, stop the reaction after continuing to stir and reflux for 24h; cool to room temperature, add methanol to settle, filter and wash, and vacuum dry at 60°C To constant weight, the yield was 89%.
[0043] 2. Continue vacuum drying the above intermediate product at 80°C for 2h, then reflux in anhydrous pyridine for 4h, add excess 4-chlorophenyl isocyanate, continue to reflux at 80°C for 14h, stop the reaction, and fully Wash and vacuum dry.
[0044] 3. The intermediate product obtained in the previous step was dissolved in a tetrahydrofuran solution containing a small amount of hydrochloric acid (1.9% by volume of tetrahydrofuran) for hydr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com