Isoindigo based co-polymer, and preparation method and application thereof
A technology of copolymer and isoindigo, which is applied in the field of solar cell materials, can solve the problems of low conversion efficiency of inorganic solar cells, low carrier electrode collection efficiency, and mismatched spectral response, so as to increase the adjustment of photoelectric performance and increase the solubility , the effect of solving the problem of low efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
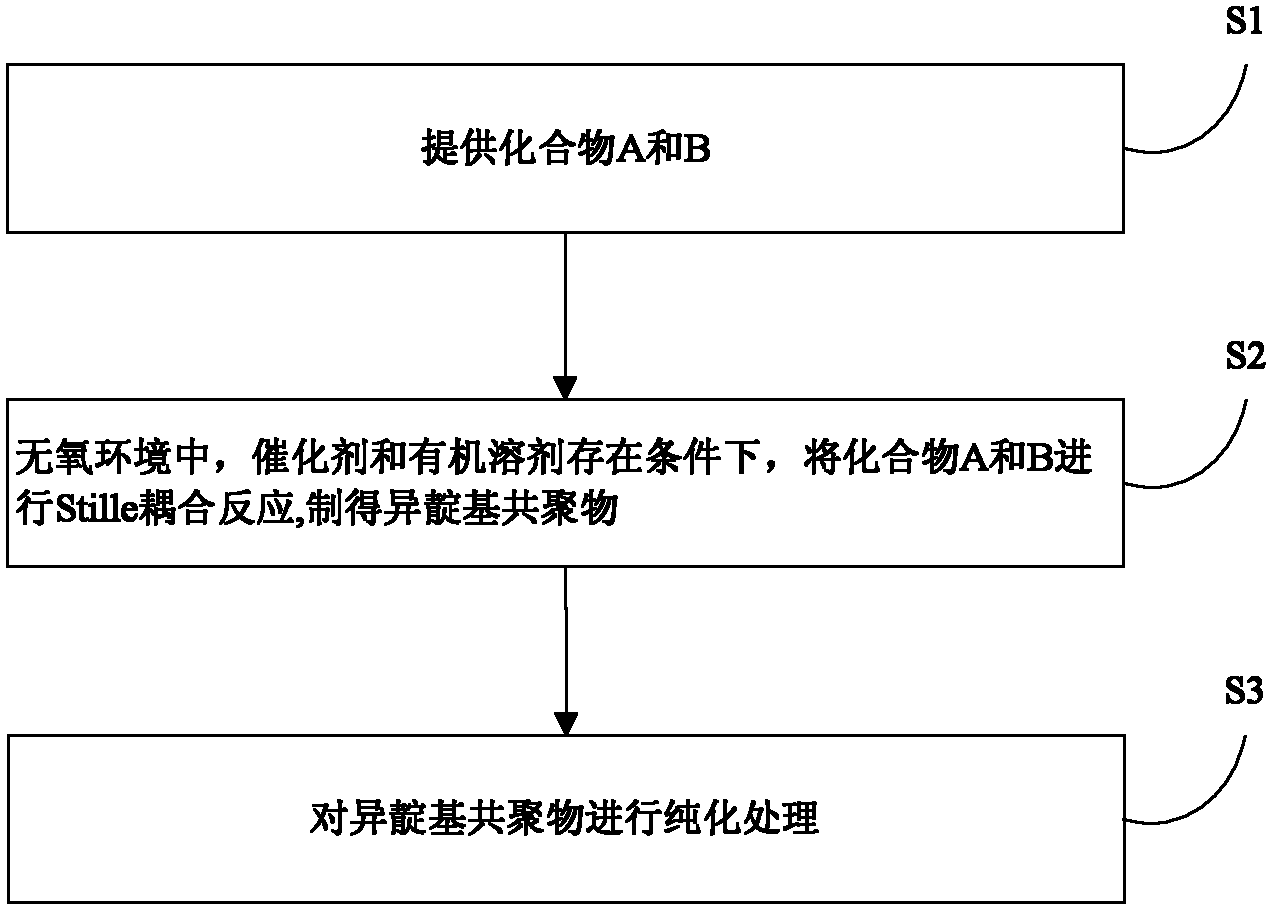
[0026] The preparation method of above-mentioned isoindigo copolymer, such as figure 1 shown, including the following steps:
[0027] S1, respectively provide compound A and compound B represented by the following structural formula,
[0028] A: 5,5'-Ditrimethyltin-4,4'-dialkyl-2,2'-bithiazole
[0029] B: 6,6'-dibromo-N,N'-dialkylisoindigo (193mg, 0.3mmol);
[0030] Among them, in compound A, R 1 for C 1 ~C 20 The alkyl group; in compound B, R 2 for C 1 ~C 20 the alkyl group;
[0031] S2. In an oxygen-free environment (such as an oxygen-free environment composed of nitrogen, argon, or a mixture of nitrogen and argon), the compound A and compound B are added in a molar ratio of 1:1 into the catalyst containing After fully dissolving in an organic solvent, carry out the Stille coupling reaction at 70-130° C. for 6-60 hours, then cool down to stop the reaction to obtain a mixed solution, which contains the product, that is, the isoindigo having the following structur...
Embodiment 1
[0045] The isoindigo copolymer of this embodiment, that is, poly{4,4'-di-n-octyl-2,2'-bithiazole-co-N,N,-di-n-octylisoindigo}, wherein, R 1 is n-octyl, R 2 Be n-octyl, n is 60, and its structural formula is as follows:
[0046]
[0047] The preparation steps of above-mentioned polymer are as follows:
[0048] The reaction formula is as follows:
[0049]
[0050] 5,5'-ditrimethyltin-4,4'-dioctyl-2,2'-bithiazole (215mg, 0.3mmol), 6,6'-dibromo-N,N'-dioctyl Diisoindigo (193mg, 0.3mmol), tridibenzylideneacetone dipalladium (13.75mg, 0.015mmol) and tri-tert-butylphosphine (24.2mg, 0.12mmol) were added into a flask containing 12mL of toluene and dissolved into a solution , fully blown nitrogen into the flask to exhaust the air for about 30 minutes, stirred at 95° C., performed Stille coupling reaction for 40 hours, and stopped the polymerization reaction after cooling down to obtain a mixed solution.
[0051] Add 40mL of methanol to the flask, carry out precipitation treatmen...
Embodiment 2
[0055] The isoindigo copolymer of the present embodiment, that is, poly{4,4'-dimethyl-2,2'-bithiazole-co-N,N'-di(n-eicosyl)isoindigo}, wherein , R 1 is methyl, R 2 It is n-eicosyl, n is 40, and its structural formula is as follows:
[0056]
[0057] The preparation steps of above-mentioned polymer are as follows:
[0058] The reaction formula is as follows:
[0059]
[0060] 5,5'-ditrimethyltin-4',4'-dimethyl-2,2'-bithiazole (104mg, 0.2mmol) and 6,6'-dibromo-N,N'-di (n-Eicosyl)isoindigo (196mg, 0.2mmol) was added into a 15ml flask of N,N-dimethylformamide, dissolved into a solution, and the flask was vacuumed to remove oxygen and filled with argon, and then added bis Triphenylphosphinepalladium dichloride (5.6mg, 0.008mmol) was stirred at 120°C for Stille coupling reaction for 12h, and the polymerization reaction was stopped after cooling down to obtain a mixed solution.
[0061] Add 50mL of methanol into the flask, carry out precipitation treatment on the mixed sol...
PUM
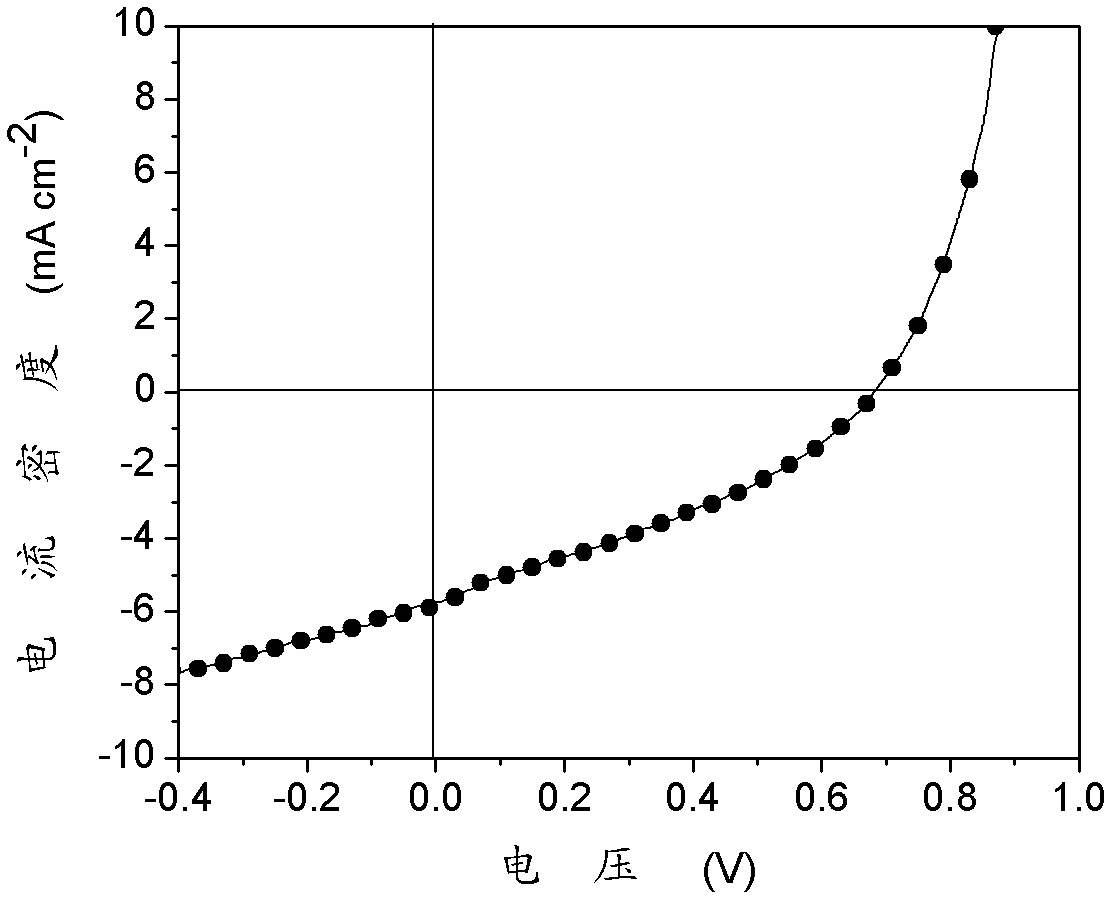
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com