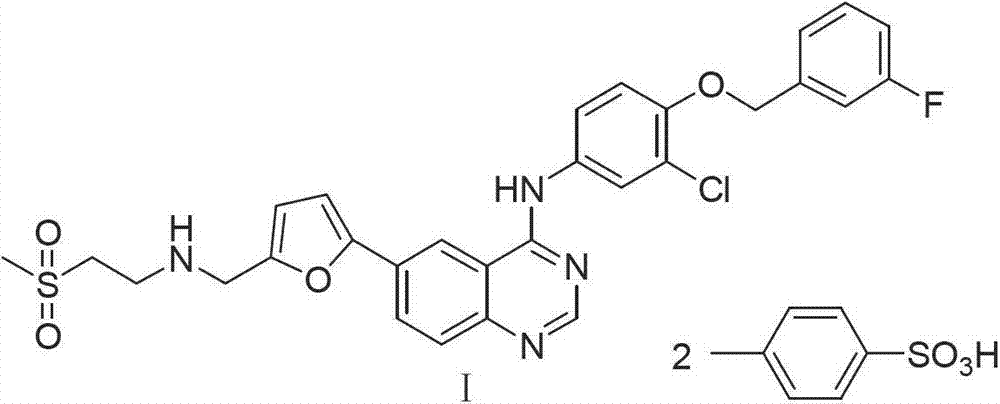
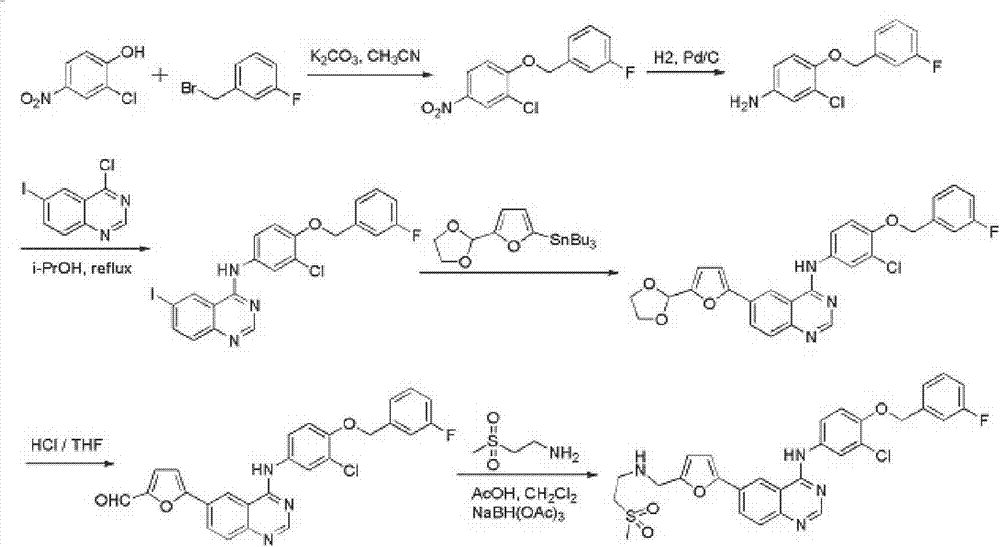
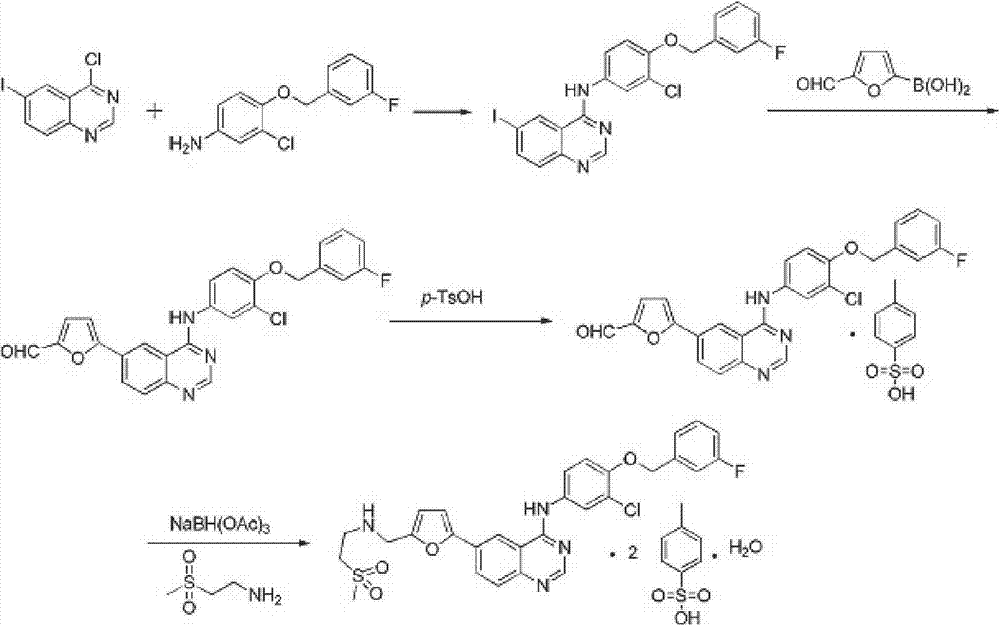
Synthetic method of lapatinib
A technology of di-p-toluenesulfonic acid and lapatinib, which is applied in the direction of organic chemistry, can solve the problems of difficult control of the reaction process of transition intermediates, poor stability of iodide intermediates, and difficulty in industrial production, so as to achieve simple reaction operation Ease of operation, easy post-processing, and shortened reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Under the protection of nitrogen, transfer 2-furfural diethyl acetal (31.6g, 2.5eq) and 310mL dry ethylene glycol dimethyl ether into the flask, cool the system down to -40°C, and add n-butyl lithium in tetrahydrofuran (106mL, 2.2mol / L, 2.5eq) was added dropwise to the reaction system, maintained at -40~-50°C and stirred for 2.5~3h, then added dropwise 51.2mL of triisopropyl borate, then stirred at -60°C for 1h Then let the system warm up to room temperature naturally, slowly add 12.8mL glacial acetic acid dropwise, then stir for 30min, then add dropwise 15.5mL water. 126 mL of methanol, 25.9 mL of triethylamine, and then N-[3-chloro-4-[(3-fluorophenyl)methoxy]phenyl]-6-iodo-4-quinazolinamine ( 37.5g, 1eq), 4.5g of 10% palladium carbon, and then the reaction system was heated to reflux temperature, and reacted for 14h. After the temperature of the reaction mixture was lowered to room temperature, it was filtered, the filter cake was washed with ethylene glycol dimethyl...
Embodiment 2
[0027]Under the protection of nitrogen, transfer 474g of 2-furfural diethyl acetal and 4500mL of dry tetrahydrofuran to the flask, cool the system to -40°C, and add a tetrahydrofuran solution (1590mL, 2.2mol / L) of n-butyllithium dropwise to In the reaction system, keep stirring at -40~-50°C for 2.5~3h, then add 768mL of triisopropyl borate dropwise, then stir at -60°C for 1h, let the system warm up to room temperature naturally, and slowly add 192mL of glacial acetic acid dropwise, Then stirred for 30min, and then added dropwise 78mL of water. Add 1890mL ethanol thereto, 388mL triethylamine, then add N-[3-chloro-4-[(3-fluorophenyl)methoxy]phenyl]-6-iodo-4-quinazolinamine 562g, 68g of 10% palladium carbon, and then the reaction system was heated to reflux for 14h. After the temperature of the reaction mixture was lowered to room temperature, it was filtered, the filter cake was washed with tetrahydrofuran, and the filtrates were combined. Add 120g of triethylamine to the filt...
Embodiment 3
[0029] Under the protection of nitrogen, 950 g of 2-furfural diethyl acetal and 9000 mL of dry tetrahydrofuran were transferred to the flask, the temperature of the system was lowered to -40 ° C, and the tetrahydrofuran solution (3180 mL, 2.2 mol / L) of n-butyllithium was added dropwise to In the reaction system, keep stirring at -40~-50°C for 2.5~3h, then add 1536mL of triisopropyl borate dropwise, then stir at -60°C for 1h, let the system warm up to room temperature naturally, and slowly add 384mL of glacial acetic acid dropwise, Then stirred for 30min, and then added dropwise 156mL of water. Add 3780mL ethanol thereto, 776mL triethylamine, then add N-[3-chloro-4-[(3-fluorophenyl)methoxy]phenyl]-6-iodo-4-quinazolinamine 1124g, 134 g of 10% palladium on carbon, and then the reaction system was heated to reflux temperature for 14 hours of reaction. After the temperature of the reaction mixture was lowered to room temperature, it was filtered, the filter cake was washed with te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com