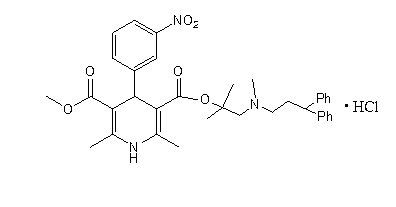
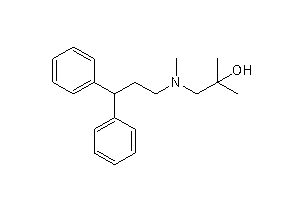
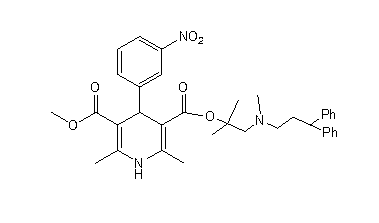
Method for scale lercanidipine hydrochloride preparation
A technology of lercanidipine hydrochloride and compound, applied in the direction of organic chemistry, etc., to achieve the effects of high purity, increased safety and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0026] (1) Dimethyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate (DHPCOOCH 3 ) preparation: Take 5g (0.033mol) of m-nitrobenzaldehyde, 8mL (0.072mol) of methyl acetoacetate, 10mL of ethanol, add it into a 100 mL three-necked flask, heat up to 58°C under stirring, and add 4g (0.05 mol) ammonium bicarbonate, 4mL water, stir until no bubbles are produced. Raise the temperature, add zeolite, stir and reflux for 5h. Cool, filter with suction, dry, and weigh 9.4g of yellow solid.
[0027] (2) Preparation of 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylic acid monomethyl ester (DHPCOOH): Take 7.0g ( Put 0.02mol) DHPCOOCH3 and 120mL methanol in a 500mL three-neck flask, stir evenly, add 6.4g (0.16mol) NaOH aqueous solution to the reaction flask after cooling, slowly heat to 75°C for reflux reaction for 5h, distill off part of the methanol under reduced pressure, and transfer to the residue Add 400mL of water, add 0.5g of activated carbon,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com