Application of stilbene glucosides in treating and preventing AIDS
A technology of stilbene glycosides and compounds, applied in medical preparations containing active ingredients, antiviral agents, organic active ingredients, etc., can solve the problem of HIV protease inhibition without related reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1HI
[0039] Expression, purification, protein activity detection and IC of embodiment 1 HIV-1 protease 50 Determination of value
[0040] (1) Amplification of HIV-1 protease fragment and construction of expression vector
[0041] Design primer PCR to amplify the HIV-1 protease coding fragment, recover the PCR product and pET-11a vector and digest it with Nde I and BamH I, recover the target fragment, connect the digested fragment with the linearized vector and transform E. coli.DH5α competent cells. The transformed clones were picked for PCR identification, and the positive clones were subjected to DNA sequencing.
[0042] Gene sequence of HIV-1 protease:
[0043] cctcagatcactctttggcaacgaccccctcgtcacaataaagataggggg
[0044] gcaactaaaggaagctctattagatacaggagcagatgatacagtattag
[0045] aagaaatgagtttgccaggaagatggaaaccaaaaatgatagggggaat
[0046] tggaggttttatcaaagtaagacagtatgatcagatactcatagaaatctg
[0047] cggacataaagctataggtacagtattaggtaggacctacacctgtcaacat
[0048] aattggaagaaa...
Embodiment 2
[0055] Example 2 Isolation of HIV-1 protease inhibitors from night-closed doors
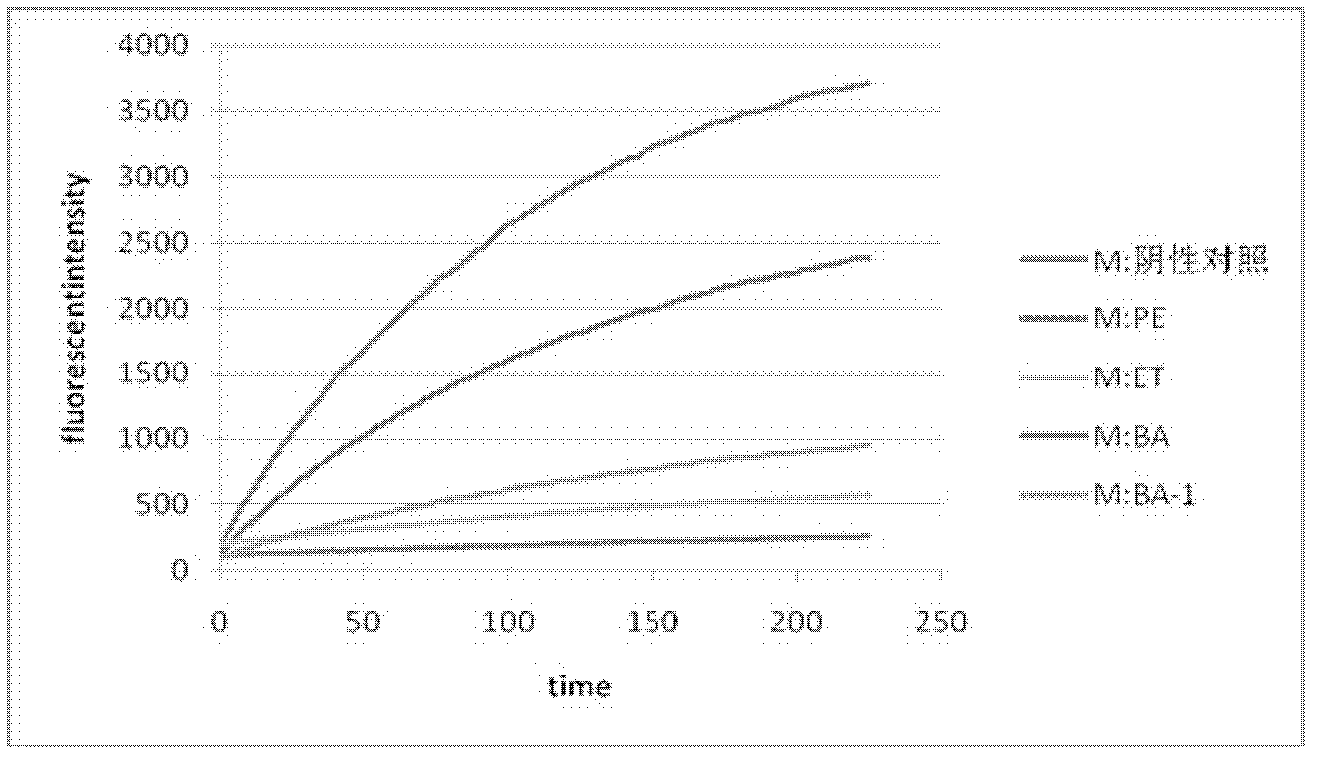
[0056] 30 grams of 95% ethanol extract of Ye Shumen was dispersed with 1.4 times the amount of double-distilled water for 2 hours by ultrasonication, poured into a separatory funnel, extracted with petroleum ether, ethyl acetate and n-butanol in turn, fully shaken, Leave to stand for 6 hours, extract each solvent 3 times, separate the organic phase and the aqueous phase, and concentrate the extract with an EYELA N1001 rotary evaporator to obtain a dry paste that is extracted from petroleum ether, ethyl acetate and n-butanol. Screening and isolation of samples as HIV protease inhibitors in vitro.
[0057] The inhibitory effect of the extracts of the above three solvents on HIV protease was tested at a drug concentration of 0.5 mg / ml, and the inhibitory rates were found to be 39.81%, 92.71% and 97.86% respectively, and the n-butanol layer was removed by polyamide chromatography. The inhibition rat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Inhibitory activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com