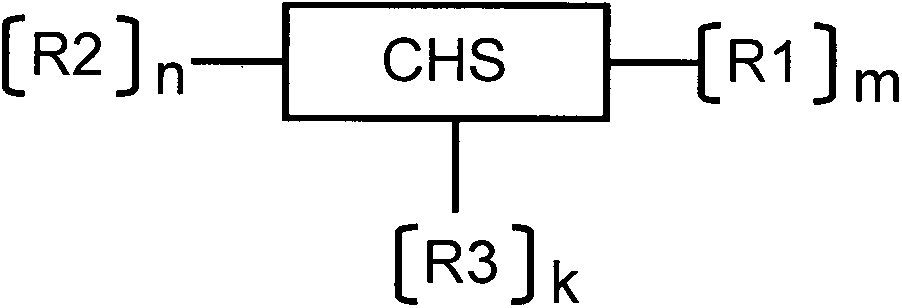
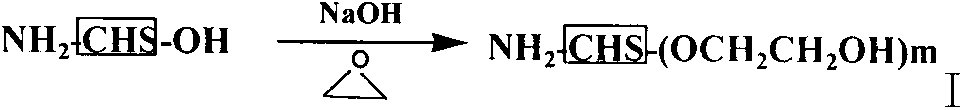
Sulfhydrylated amphipathic chitosan polymer carrier as well as preparation method and application thereof
A chitosan derivative, amphiphilic technology, applied in the field of pharmaceutical preparations, can solve problems such as poor solubility and oral administration restrictions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Embodiment 1: Preparation of 6-O-carboxymethyl-2-N-octyl-2-N-mercaptoacetyl chitosan
[0088] 1. Preparation of 6-O-carboxymethyl-2-N-octyl chitosan
[0089] 1.1 Preparation of 16-O-carboxymethyl chitosan
[0090] Dissolve 1g of chitosan in acetic acid aqueous solution, stir to dissolve, then add an equal volume of isopropanol, add NaOH in batches, alkalinize in a water bath at 40°C, dissolve 6g of chloroacetic acid in isopropanol, add dropwise to the reaction solution, and react at 40°C After a certain period of time, pour off the supernatant and add 40mL of water to make it a clear and transparent yellow solution. Adjust the pH of the solution to neutral with HAC, disperse in methanol, filter, wash the filter cake with methanol / water (90:10, v:v) several times, dissolve the filter cake in 20 mL of water, filter, and lyophilize to obtain 6-O - Carboxymethyl chitosan.
[0091] 1.2 Preparation of 6-O-carboxymethyl-2-N-octyl chitosan
[0092] 6-O-carboxymethyl chitosan ...
Embodiment 2
[0096] Embodiment 2: Preparation of 6-O-hydroxyethyl-2-N-deoxycholic acid-2-N-(N-acetyl-L-cysteine) chitosan
[0097] 1. Preparation of 6-O-hydroxyethyl-2-N-deoxycholic acid chitosan
[0098] 1.1 Preparation of 16-O-hydroxyethyl chitosan
[0099] Chitosan 1g, suspended in 15ml 2% HAC, dropwise added 50% NaOH 10ml, alkalized in water bath at 40°C for 12h, added dropwise ethylene oxide in ice bath, stirred in ice bath for 2-6h, condensed and refluxed, heated to 30-50°C Continue to react at ℃, pour off the supernatant, add a certain amount of water, adjust to neutral with 5M HCl, transfer to a dialysis bag (MWCO=12000-14000), dialyze with distilled water overnight, and freeze-dry the dialyzate to obtain 6-O - Hydroxyethyl chitosan.
[0100] 1. Preparation of 26-O-hydroxyethyl-2-N-deoxycholic acid chitosan
[0101] 0.5 g of 6-O-hydroxyethyl chitosan was dissolved in distilled water, after an appropriate amount of deoxycholic acid, 1.5 times the molar ratio (compared with deoxyc...
Embodiment 3
[0105] Embodiment 3: Preparation of 6-O-carboxymethyl-2-N-cholic acid-2-N-glutathione chitosan
[0106] 1. Preparation of 6-O-carboxymethyl-2-N-cholic acid chitosan
[0107] 1.1 Preparation of 16-O-carboxymethyl chitosan
[0108] Method is the same as 1.1 in Example 1.
[0109] 1. Preparation of 26-O-carboxymethyl-2-N-cholic acid chitosan
[0110] Dissolve 0.5 g of 6-O-carboxymethyl chitosan in distilled water, dissolve an appropriate amount of cholic acid, 1.5 times the molar ratio (compared to cholic acid) of EDC and NHS in methanol, add it to the reaction solution, and stir to react After 24 hours, evaporate at 40° C., adjust the pH to 7 with 5% NaOH, dialyze and freeze-dry to obtain 6-O-carboxymethyl-2-N-cholic acid chitosan.
[0111] 1.3 The reaction sequence of the above two steps can be reversed, that is, cholic acid is first introduced into the chitosan skeleton, and then carboxymethyl is introduced.
[0112] 2. Preparation of 6-O-carboxymethyl-2-N-cholic acid-2-N-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com