Novel drug resistant gene of Klebsiella pneumoniae
A technology of bacteria and drugs, applied in the field of new drug-resistant genes of Klebsiella pneumoniae
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Discovery of new resistance gene KPC-15
[0026] -Materials and methods
[0027] 1. Materials
[0028] VITEK2 from French bioMerieux; bacterial identification card; VITEK2GN Test kit; bacterial susceptibility card; AST-GN13 from French bioMerieux; supplementary susceptibility paper from Oxoid Ltd, UK (75μg / 30μg cefoperazone / sulbactam, 30μg minocycline Element); miniBEST plasmid purification Kit from TakaRa company; high-fidelity polymerase, other reagents are commercially available.
[0029] 2. Method
[0030] 2.1 Isolation and identification of Klebsiella pneumoniae KP1241 strain
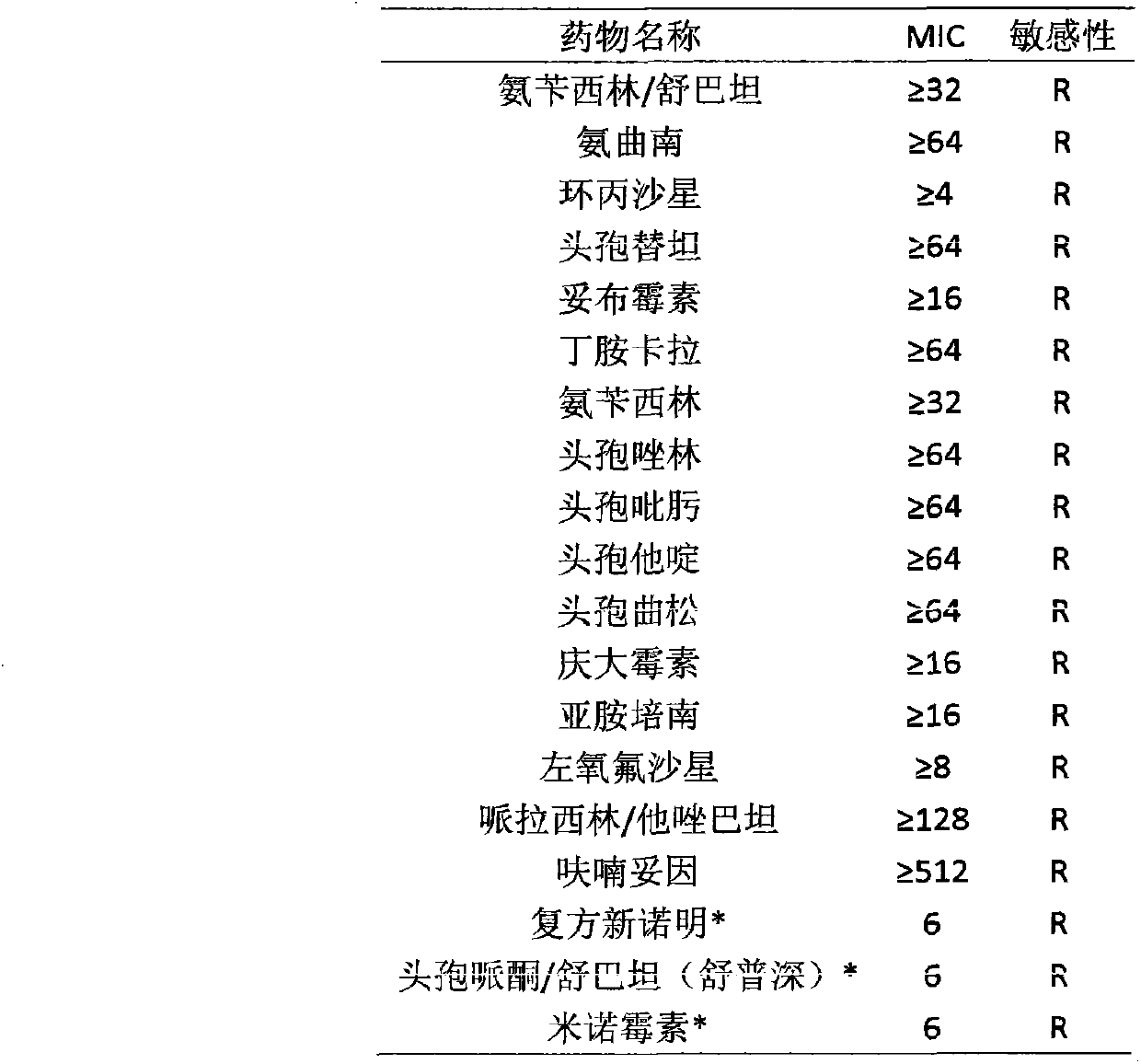
[0031] The blood culture-positive strains from patients in the cardiothoracic surgery department of Taizhou Municipal Hospital were transferred to blood plates for isolation and culture, and cultured at 35°C in a 5% CO2 incubator for 16-18 hours. Bacterial identification and drug susceptibility analysis system and drug susceptibility plate agar diffusion method (supplement drug s...
Embodiment 2
[0068] Example 2 Bacterial plasmid transduction experiment and drug resistance detection
[0069] 1. Materials and methods
[0070] 1. Materials
[0071] Klebsiella pneumoniae (accession KP1241); wild type E. coli J53.
[0072] 2. Methods and results
[0073] Klebsiella pneumoniae KP1241 and wild-type E.coli J53AzR (sodium azide resistance) were used as donor and recipient bacteria respectively, and 0.5ml of each in the logarithmic growth phase was added to 4ml of fresh LB Incubate overnight in broth. Conjugates were screened on tryptic soy agar (TSA) plates containing sodium azide (300 mg / L) and ciprofloxacin (0.03 mg / L), and incubated at 35°C for 18-24 hours. The screened zygotes were analyzed for their sensitivity to drugs with bioMerieux's VITEK2 bacterial identification and drug susceptibility analysis system and drug susceptibility plate agar diffusion method (supplemented with drug susceptibility discs). The results are shown in Table 2:
[0074] Table 2E.coliJ53Az...
Embodiment 3
[0078] Example 3 Construction of constitutive KPC-15 gene expressing bacteria
[0079] 1. Materials and methods
[0080] 1. Materials
[0081] E.coli JM109; pMD18T vector from TaKaRa company; pBR322 vector; restriction endonucleases Pst I and EcoR I from TaKaRa company; agarose gel recovery kit from Tiangen Biochemical Technology (Beijing) Co., Ltd.; TaKaRa company's T4 DNA ligase.
[0082] 2. Method
[0083] 2.1 Construction of constitutive KPC-15 gene expressing strain E.coli JM109 (pET32a-KPC-15)
[0084] The PCR product in Example 1 was ligated with the pMD18T vector, and the ligated product was added to 100 μl of JM109 competent cells for transformation, cultured on a plate containing IPTG, x-gal, and Amp, and the gene sequence and its gene insertion direction were verified by sequencing. Then the KPC-15 gene was cloned into the pET32a vector to obtain the pET32a-KPC-15 plasmid containing the KPC-15 gene. The recombinant plasmid was transformed into competent E.coli ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com