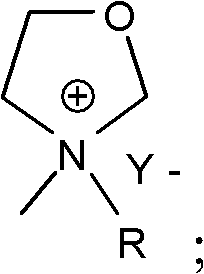
Oxazolidines ionic liquid containing carbonic ester perssad and preparation method and application thereof
A carbonate-based, ionic liquid technology, used in organic chemistry, capacitors, circuits, etc., can solve problems such as slow research progress, and achieve the effects of simple preparation process, improved performance, good dissolution and dissociation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] The preparation method of the above-mentioned oxazolidine ionic liquid containing carbonate group, such as figure 1 shown, including the following steps:
[0021] S1, carry out synthetic reaction with the N-methyl oxazolidine that molar ratio is 1: 1.1~1.2 and RX (being halogenated carbonic acid ester), the obtained structural formula after reaction finishes is N-methylazolidine halides containing carbonate groups (or called N-methylazolidine halides containing carbonate groups); wherein, R is 0≤n≤4; X is a halogen element, selected from Cl or Br; the reaction formula is as follows:
[0022]
[0023] Among them, RX is Selected from chloroethylene carbonate, chloropropylene carbonate, chlorobutylene carbonate, chloropentenyl carbonate, chlorohexenyl carbonate, bromoethylene carbonate, bromopropylene carbonate, bromocarbonic acid Butenyl ester, bromopentenyl carbonate or bromohexenyl carbonate, etc.; when n is 0, it is chloroethylene carbonate; when n is 4, it is...
Embodiment 1
[0035] Example 1 Synthesis of N-propylene carbonate-N-methylazolidine tetrafluoroborate
[0036] 1. In a 250mL flask, add (87g, 1mol) N-methylazolidine and (149.6g, 1.1mol) chloropropylene carbonate respectively; 2 Under the protection of the atmosphere, the temperature was raised to 60°C, and the synthesis reaction was stirred for 72h; then it was left to cool, and the resultant was washed with ethyl acetate three times, and vacuum-dried at 80°C to obtain light yellow N-propylene carbonate-N- Methylazolidine chloride salt, the yield is 75%;
[0037] 2. The N-propylene carbonate-N-methylazolidine chloride salt (111.5g, 0.5mol) prepared above, NaBF 4 (or KBF 4 ) (55g, 0.5mol) and 100mL of deionized water were added to a 500mL flask, and the reaction was stirred at room temperature for 10h; after the reaction was completed, a mixed solution was obtained; then, the mixed solution was extracted 3 times with 250mL of dichloromethane, and the extracts were combined; Then back-ext...
Embodiment 2
[0040] Example 2 Synthesis of N-vinyl carbonate base-N-methylazolidine hexafluorophosphate
[0041] 1. In a 250mL flask, add (87g, 1mol) N-methylazolidine and (182.6g, 1.1mol) bromoethylene carbonate respectively; 2 Under the protection of the atmosphere, the temperature was raised to 80°C, and the synthesis reaction was stirred for 48h; then it was left to cool, washed with ethyl acetate three times, and dried in vacuum at 80°C to obtain light yellow N-vinyl carbonate-N-methyl Azolidinium bromide, the yield is 74%;
[0042] 2. The N-vinyl carbonate base-N-methylazolidine bromide (126.5g, 0.5mol) and KPF 6 (92g, 0.5mol) and 150mL of deionized water were added to a 500mL flask, and the ion exchange reaction was stirred at room temperature for 24h; after the reaction was completed, the mixed solution of or; then, the mixed solution was extracted 3 times with 250mL of dichloromethane, and the extracts were combined ; Then back-extract with 60mL deionized water each time until s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com