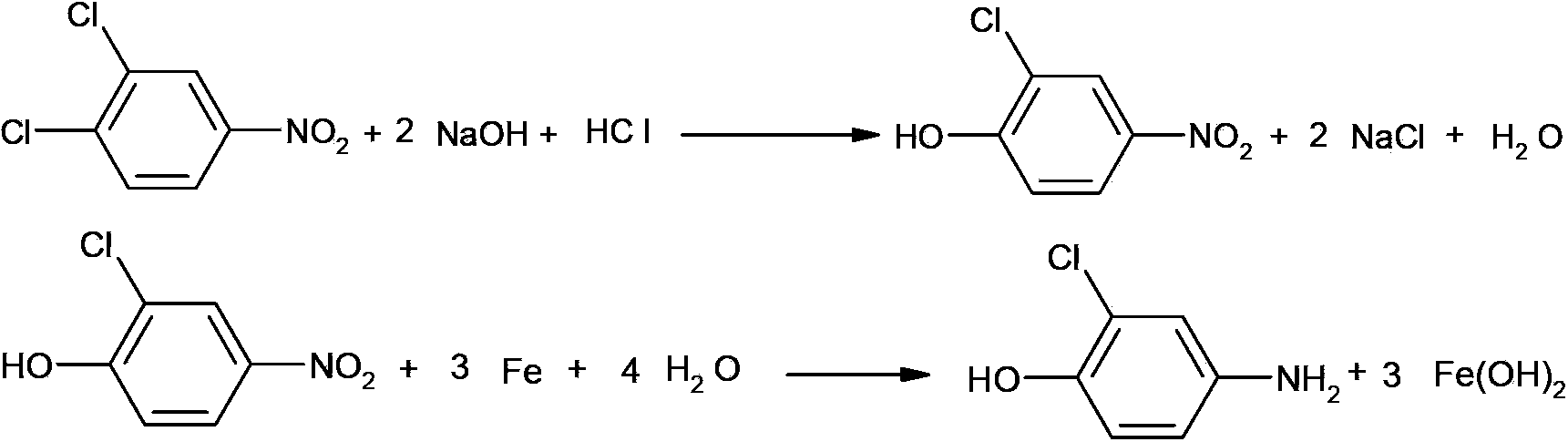
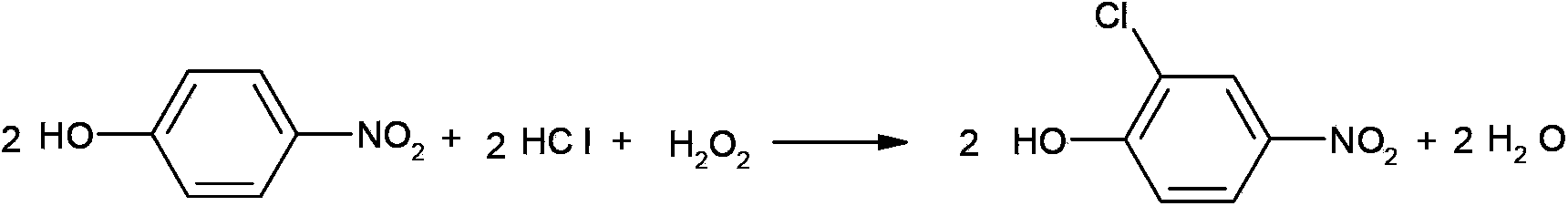
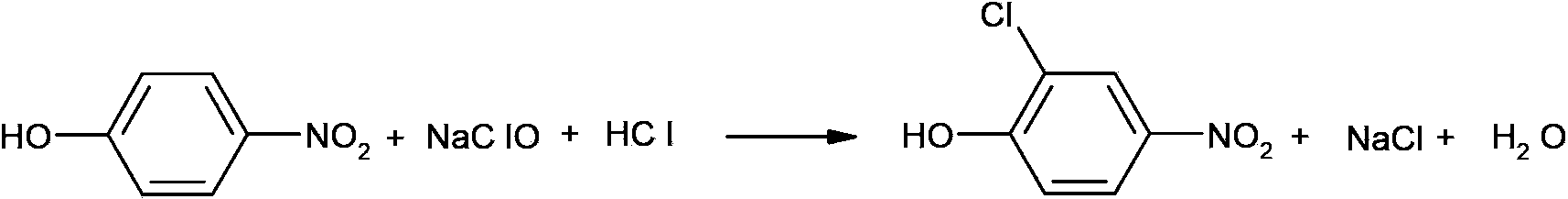
Synthetic method of 2-chloro-4-aminophenol
A synthesis method and aminophenol technology are used in the synthesis of pesticide intermediates, and in the field of synthesis of 2-chloro-4-aminophenol, which can solve reaction difficulties, low yield of 2-chloro-4-aminophenol, and low yield of 2-chloro-4-aminophenol. There are many problems such as by-products and large pollution, so as to achieve the effect of saving synthesis cost, high purity and low pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment l
[0040] The synthesis of embodiment 1,2-chloro-4-nitrophenol
[0041] Add 60 grams of 99% p-nitrophenol (0.427 moles) and 240 grams of solvent dichloroethane in a 500 milliliter four-necked bottle equipped with a thermometer, agitator, reflux condenser and tail gas absorption device, stir and heat to 55-65°C, open the vacuum tail gas absorption system to ensure that the condition is slightly negative -0.01-0.02MPa, then pass chlorine gas, use GC to track the reaction process, and wait for the reaction of raw materials to complete. Firstly, recover the solvent dichloroethane from atmospheric pressure precipitation and apply it directly, and then distill the residual solvent dichloroethane under reduced pressure at 10mmHg to recover the cover steam to obtain the crude product o-chloro-p-nitrophenol, lower the temperature to below 50°C, add 200 grams of toluene, and heat up After complete dissolution, slowly cool down to natural precipitation, then cool down in an ice bath to (0-5...
Embodiment 2
[0042] The synthesis of embodiment 2,2-chloro-4-aminophenol
[0043] Add 107 grams of o-chloro-p-nitrophenol (0.62 moles) and 210 grams of water, 4.9 grams of activated carbon and 1.2 grams of Ferric chloride hexahydrate, add 12.4 grams of 30% sodium hydroxide (0.093 moles) solution dropwise. Let the temperature rise naturally, and the drop will be completed in about 30 minutes. Make the raw material fully contact with the catalyst. Add 128 grams of 40% hydrazine hydrate (1.023 moles) dropwise at 95-100°C, keep the temperature for 3 hours after dropping, after the reaction is completed, the reaction solution is cooled to 40-45°C and filtered, and the filter residues are washed twice with 100 ml of water, combined After the filtrate, 30% concentrated hydrochloric acid was added dropwise until neutral, and the precipitated light yellow solid was filtered and dried to obtain 85.0 g of 2-chloro-4-aminophenol, with a qualitative content of 98.2% and a qualitative yield of 93.8%. ...
Embodiment 3
[0044] Embodiment 3, catalyst recovery is used for the synthesis of 2-chloro-4-aminophenol
[0045] Add 107 grams of o-chloro-p-nitrophenol (0.62 mole), 210 grams of water, 5.3 grams of reclaimed catalyst (activated carbon and six After stirring, add 10.7 grams of 30% sodium hydroxide (0.080 mol) solution dropwise. After about 30 minutes, the temperature is raised to 95-100°C and kept at this temperature for 0.5 hours, so that the raw materials and Catalyst full contact. Add 128 grams of 40% hydrazine hydrate (1.023 moles) dropwise at 95-100°C, and keep the temperature for 3 hours after dropping. After the reaction is completed, the reaction solution is cooled to 40-45°C and filtered, and the filter residues are washed twice with 100 ml of water. After combining the filtrates, 30% concentrated hydrochloric acid was added dropwise until neutral, and the precipitated light yellow solid was filtered and dried to obtain 84 grams of 2-chloro-4-aminophenol, with a qualitative conte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com