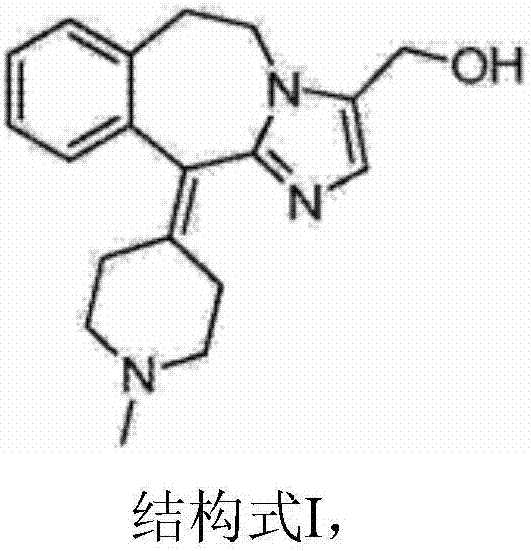
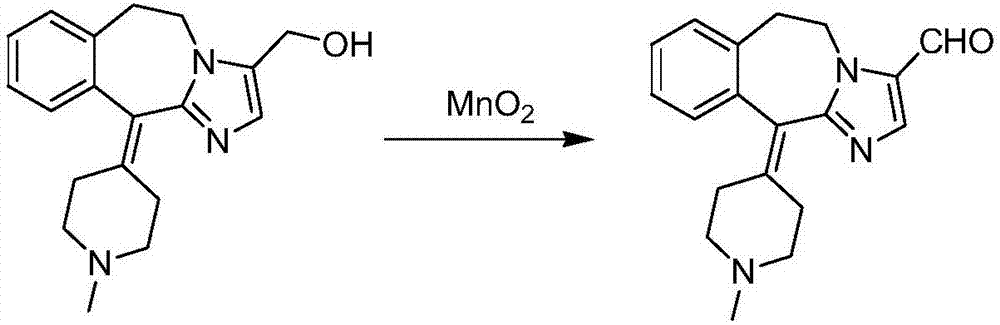
A kind of oxidation method for preparing alcatadine
A technology for alcaftadine and intermediates, applied in the field of compound preparation, can solve the problems of unsuitability for industrial scale-up production, polycarboxylic acid impurities, high toxicity, etc., and achieve poor product purity, high total yield, and less impurities Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
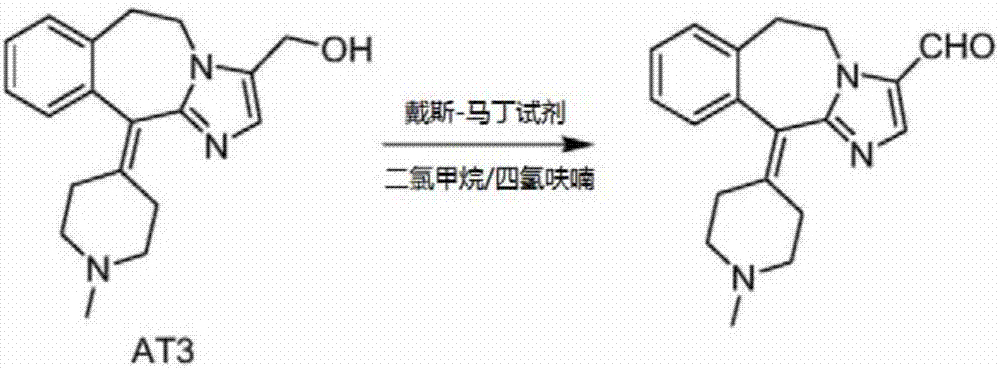
[0026] Add 30.9g (0.1mol) of AT3, 300mL of dichloromethane, and 150mL of tetrahydrofuran into a 1000mL three-neck flask, stir and dissolve at 10-20°C, add 84.8g (0.2mol) of Dess-Martin reagent, and heat up to 20-20°C after the addition is complete. Continue stirring at 30°C for 1-2 hours, monitor by TLC until the reaction is complete, filter with suction, wash the filtrate once with 100 mL of 10% sodium thiosulfate solution, separate the organic layer, then wash with 100 mL of saturated 5% sodium bicarbonate solution, separate The organic layer was taken out, dried by adding anhydrous sodium sulfate, filtered with suction, and the filtrate was concentrated under reduced pressure. Add 50 mL of isopropanol to the obtained oil and stir, cool down to precipitate a solid, filter and dry to obtain 27.5 g of off-white solid, yield 89.6%. HPLC purity: 98.5%.
Embodiment 2
[0028] Add 30.9g (0.1mol) of AT3, 300mL of dichloromethane, and 150mL of tert-butanol into a 1000mL three-neck flask, stir and dissolve at 10-20°C, add 63.6g (0.15mol) of Dess-Martin reagent, and heat up to Continue stirring at 20-30°C for 1-2 hours, monitor by TLC until the reaction is complete, filter with suction, wash the filtrate once with 100 mL of 10% sodium thiosulfate solution, separate the organic layer, and wash with 100 mL of saturated 5% sodium bicarbonate solution , the organic layer was separated, dried by adding anhydrous sodium sulfate, filtered with suction, and the filtrate was concentrated under reduced pressure. The obtained oil was added with 50 mL of isopropanol and stirred, the temperature was lowered to precipitate a solid, which was filtered and dried to obtain 26.8 g of an off-white solid with a yield of 87.3%. HPLC purity: 98.6%.
Embodiment 3
[0030] Add 30.9g (0.1mol) of AT3, 200mL dichloromethane, and 200mL acetone into a 1000mL three-neck flask, stir and dissolve at 10-20°C, add 84.8g (0.2mol) of Dess-Martin reagent, and heat up to 20-20°C after adding Continue stirring at 30°C for 1-2 hours, monitor by TLC until the reaction is complete, filter with suction, wash the filtrate once with 100 mL of 10% sodium thiosulfate solution, separate the organic layer, then wash with 100 mL of saturated 5% sodium bicarbonate solution, separate The organic layer was taken out, dried by adding anhydrous sodium sulfate, filtered with suction, and the filtrate was concentrated under reduced pressure. The obtained oil was added with 50 mL of isopropanol and stirred, the temperature was lowered to precipitate a solid, and it was filtered and dried to obtain 26.5 g of an off-white solid with a yield of 86.3%. HPLC purity: 98.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com