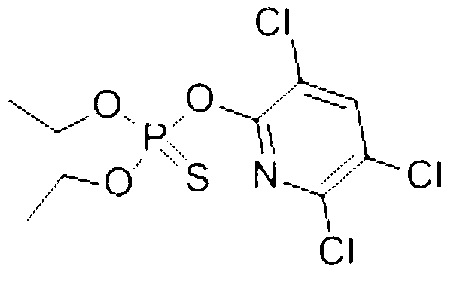
Water-based new preparation formulation ZW of chlorpyrifos, and processing and application thereof
A technology of chlorpyrifos and a new dosage form, which is applied in the field of microcapsule suspension-emulsion in water and its processing, can solve the problems of low content of active ingredients, adverse environmental impact, environmental hazards, etc., increase the content of active ingredients, and solve the problem of poor persistence , the effect of reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
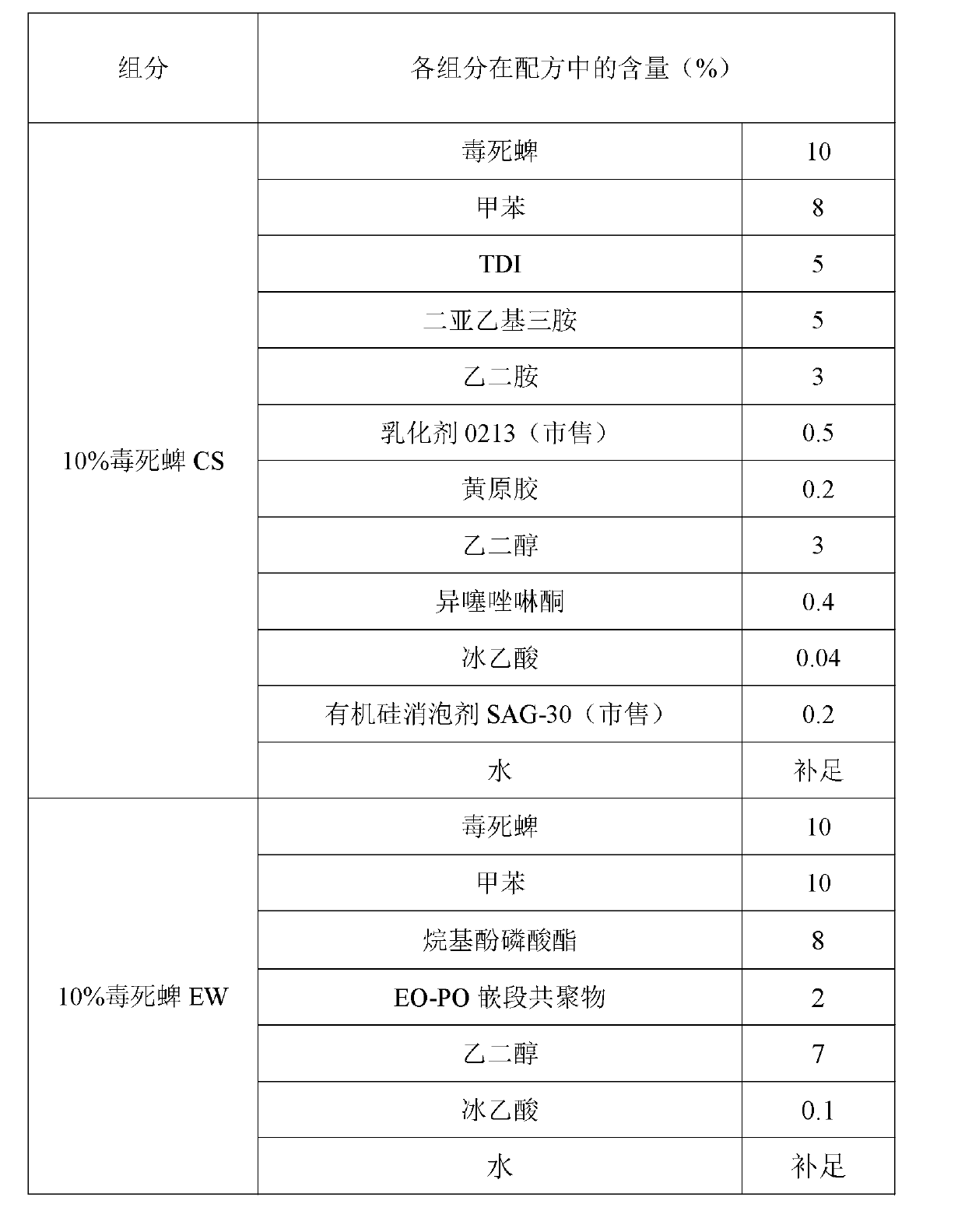
[0043] The processing method of the new dosage form of chlorpyrifos ZW in this example is as follows: first, 10% chlorpyrifos CS is processed by the interface polymerization method, and 10% chlorpyrifos EW is processed by the conventional method, and the two are uniformly mixed according to the mass ratio of 3:7 to obtain 10% chlorpyrifos ZW.
[0044] Table 1: Mixture of chlorpyrifos microcapsule suspension processed by interfacial polymerization and aqueous emulsion processed by conventional method
[0045]
[0046] The prepared 10% chlorpyrifos new dosage form ZW can meet the following technical indicators: suspension rate ≥ 93%; emulsion stability qualified; cyst formation rate ≥ 91%; automatic dispersibility ≥ 90%; pH value qualified; cold storage indicators Qualified; all indicators of heat storage are qualified; all indicators of freeze-thaw stability are qualified.
Embodiment 2
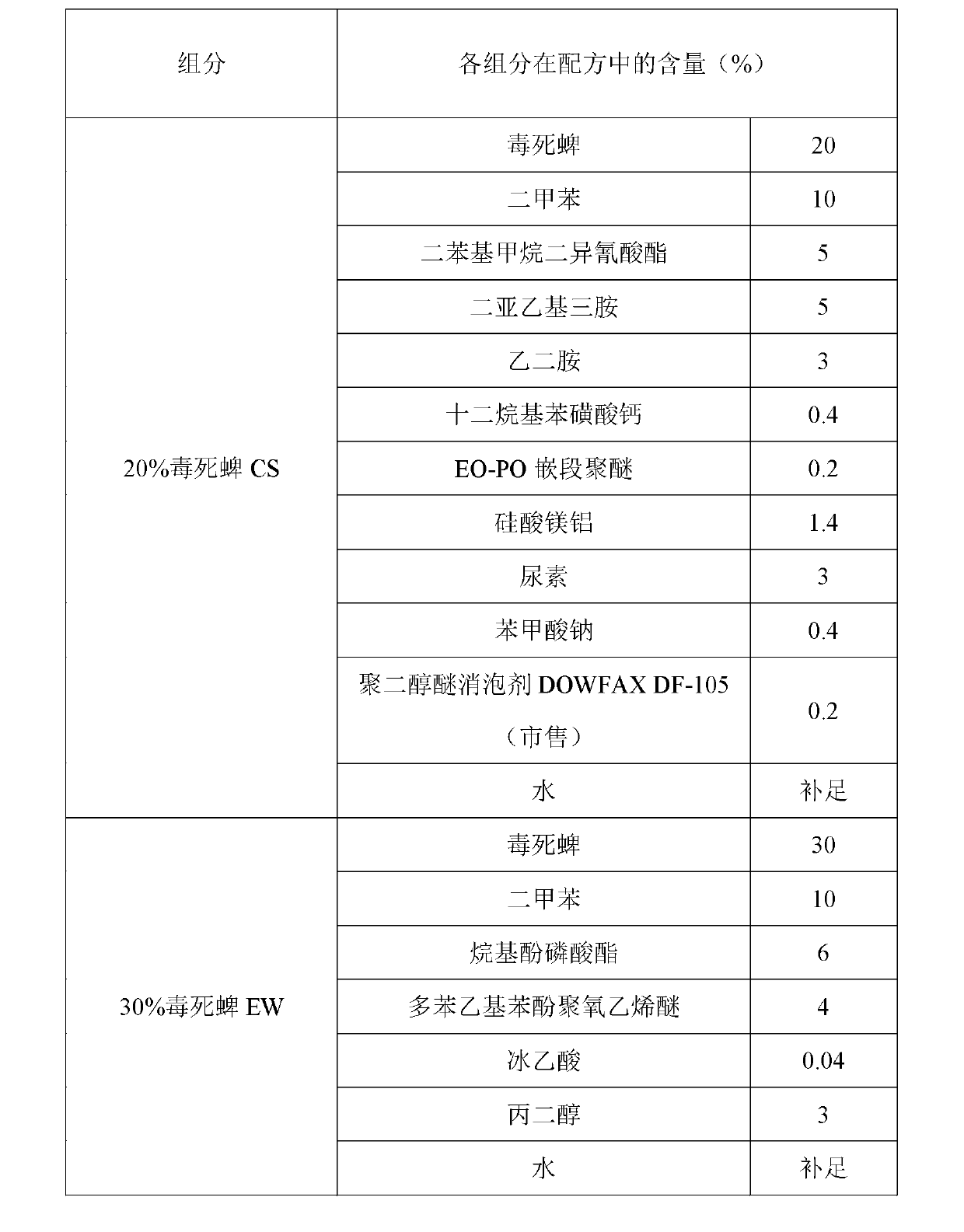
[0048] The processing method of the new dosage form of chlorpyrifos ZW in this example is as follows: first, 20% chlorpyrifos CS is processed by the interfacial polymerization method, and 30% chlorpyrifos EW is processed by the conventional method, and the two are uniformly mixed according to the mass ratio of 7:3 to obtain 23% chlorpyrifos ZW.
[0049] Table 2: Mixture of chlorpyrifos microcapsule suspension processed by interfacial polymerization and aqueous emulsion processed by conventional method
[0050]
[0051] The prepared 23% chlorpyrifos new dosage form ZW can meet the following technical indicators: suspension rate ≥ 94%; emulsion stability qualified; cyst formation rate ≥ 90%; automatic dispersibility ≥ 91%; pH value qualified; cold storage indicators Qualified; all indicators of heat storage are qualified; all indicators of freeze-thaw stability are qualified.
Embodiment 3
[0053] The processing method of the new dosage form of chlorpyrifos ZW in this example is as follows: firstly, the interfacial polymerization method is used to process chlorpyrifos CS, and after the CS is formed into capsules and solidified, the oil phase of chlorpyrifos (including chlorpyrifos, toluene, and emulsifier) is added, and the proportions are shown in the table below. 30% Chlorpyrifos ZW.
[0054] Table 3: Formula ratio of 30% chlorpyrifos ZW dosage form
[0055]
[0056] The prepared 30% chlorpyrifos new dosage form can meet the following technical indicators: suspension rate ≥ 94%; emulsion stability qualified; cyst formation rate ≥ 90%; automatic dispersibility ≥ 91%; pH value qualified; cold storage indicators qualified All indexes of heat storage are qualified; all indexes of freeze-thaw stability are qualified.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com