Preparation method of amino borane
A technology of aminoborane and synthesis method, which is applied in the field of preparation of hydrogen energy carriers, can solve problems such as environmental pollution and cost, and achieve the effects of high purity, high yield, and mild temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 1) Weigh 0.15mol of sodium borohydride and methylamine hydrochloride respectively, then put the mixture of methylamine hydrochloride and sodium borohydride into a 500mL two-neck round bottom flask, put a magnet and measure 200mL of tetrahydrofuran Add it into a round bottom flask, control the reaction temperature at 25°C, and react for 12 hours to obtain a reaction mixture solution. The reaction mixture solution is filtered under reduced pressure to obtain a filtrate, and then tetrahydrofuran is removed at room temperature to obtain the initial product.
[0021] 2) Dissolve the obtained primary product in anhydrous ether, stir in an ice-water bath for 1 h, and then filter under reduced pressure to obtain the filtrate. After removing the ether, a white crystalline solid is obtained, which is methylaminoborane.
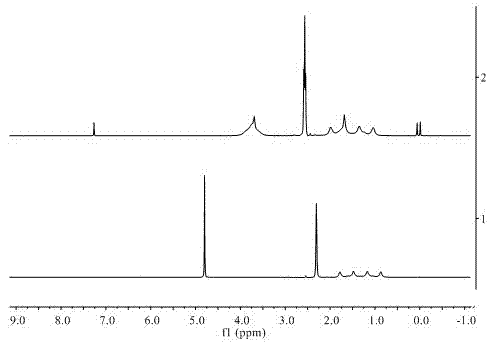
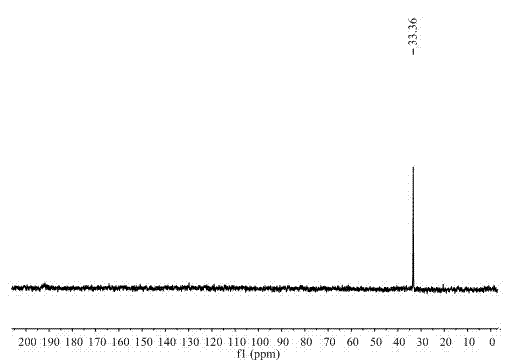
[0022] 3) Purity testing
[0023] The quality of the obtained product was claimed to be 4.3 g, and the calculated yield was 70%. Then by using RuCl 3 ·3H 2 O ...
Embodiment 2
[0028] 1) Weigh 0.005mol of sodium borohydride and methylamine hydrochloride respectively, then put the mixture of methylamine hydrochloride and sodium borohydride into a 100mL two-neck round bottom flask, put a magnet, and then measure Add 10ml of tetrahydrofuran into a round bottom flask, control the reaction temperature at 25°C, and the reaction time is 2h, 4h, 6h, and 24h respectively to obtain a reaction mixture solution, and obtain a filtrate by filtering the reaction mixture solution under reduced pressure, and then at room temperature Removal of tetrahydrofuran gave the crude product.
[0029] 2) Dissolve the obtained primary product in anhydrous ether, stir in an ice-water bath for 1 h, then filter under reduced pressure to obtain the filtrate, remove the ether, and obtain a white crystalline solid that is methylaminoborane.
[0030] 3) Purity testing
[0031] It is claimed that the amount of methylaminoborane obtained when the reaction time is 2h, 4h, 6h and 24h is ...
Embodiment 3
[0034] 1) Weigh 0.005mol of sodium borohydride and dimethylamine hydrochloride respectively, then put the mixture of dimethylamine hydrochloride and sodium borohydride into a 100mL two-necked round bottom flask, put a magnet, and measure Take 10ml of tetrahydrofuran and add it into a round bottom flask, control the reaction temperature at 25°C, and react for 12 hours to obtain a reaction mixture solution. The reaction mixture solution is filtered under reduced pressure to obtain a filtrate, and then tetrahydrofuran is removed at room temperature to obtain the initial product.
[0035] 2) Dissolve the obtained primary product in anhydrous ether, stir in an ice-water bath for 1 h, and then filter under reduced pressure to obtain the filtrate. After removing the ether, a white crystalline solid is obtained, which is dimethylaminoborane.
[0036] 3) Purity testing
[0037] The quality of the product was weighed to be 0.15 g of dimethylaminoborane, the yield was 50.76%, and the purit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com