Method for preparing urea ardealite by solution crystallization method taking ardealite as raw material
A technology of solution crystallization and phosphogypsum, which is applied in applications, fertilizer mixtures, fertilization devices, etc., can solve problems such as high moisture content, and achieve the effects of uniform particle size, precise and adjustable purity, and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
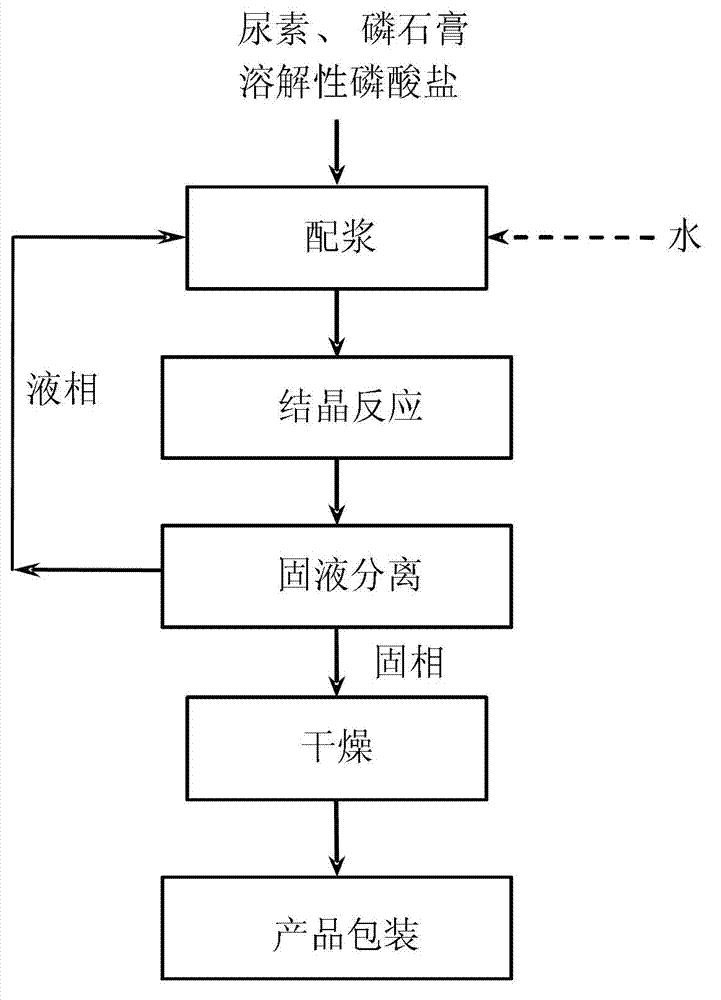
[0044] according to figure 1 In the process flow shown, under the condition of 20°C, add urea and water into the slurry mixing tank to prepare a saturated urea solution. Add phosphogypsum with a mass molar ratio of 0.9:1.0:0.1 to the saturated urea solution (based on CaSO in phosphogypsum 4 .2H 2 O meter), urea (dosing urea), ammonium dihydrogen phosphate to form a slurry of phosphogypsum and urea. Pump the slurry into the crystallization reactor, continue to stir evenly, maintain the temperature at 20°C, and stay for 10.0 hours to complete the reaction of phosphogypsum, ammonium dihydrogen phosphate and urea and convert it into urea phosphogypsum slurry, and pump the urea phosphogypsum slurry to vacuum Belt filter for solid-liquid separation. The solid phase was sent to a fluidized bed dryer for drying at 60° C. for 5 minutes to obtain urea phosphogypsum with a purity of 53.2%. After packaging, it is a urea phosphogypsum product. The separated liquid phase is returned to...
Embodiment 2
[0047] according to figure 1 In the process flow shown, under the condition of 30°C, add urea and water into the slurry mixing tank to prepare a saturated urea solution. Phosphogypsum with a mass molar ratio of 0.8:2.0:0.2 was added to the saturated urea solution (based on CaSO in phosphogypsum 4 .2H 2 O meter), urea (the urea added), sodium dihydrogen phosphate to form a slurry of phosphogypsum and urea. Pump the slurry into the crystallization reactor, continue to stir evenly, maintain the temperature at 30°C, and stay for 8.0 hours to complete the reaction of phosphogypsum, sodium dihydrogen phosphate and urea and convert it into urea phosphogypsum slurry, and pump the urea phosphogypsum slurry to vacuum Belt filter for solid-liquid separation. The solid phase was sent to a fluidized bed dryer for drying at 65° C. for 4 minutes to obtain urea phosphogypsum with a purity of 68.2%, which was packaged as a urea phosphogypsum product. The separated liquid phase is returned ...
Embodiment 3
[0050] according to figure 1 In the process flow shown, under the condition of 40°C, add urea and water into the slurry mixing tank to prepare a saturated urea solution. Add phosphogypsum with a mass molar ratio of 0.7:3.0:0.3 to the saturated urea solution (based on CaSO in phosphogypsum 4 .2H 2 O meter), urea (dosing urea), potassium dihydrogen phosphate to form a slurry of phosphogypsum and urea. The slurry is pumped into the crystallization reactor, continuously stirred evenly, the temperature is maintained at 40°C, the residence time is 6.0h, the reaction of phosphogypsum, potassium dihydrogen phosphate and urea is completed and converted into urea phosphogypsum slurry, and the urea phosphogypsum slurry is pumped to the centrifuge Filter for solid-liquid separation. The solid phase is sent to a fluidized bed dryer for drying at 70° C. for 3 minutes to obtain urea phosphogypsum with a purity of 79.5%, which is packaged as a urea phosphogypsum product. The separated liq...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com