Imatinib mesylate tablet cores, coated tablets, and preparation method thereof
A technology of imatinib mesylate and coated tablets is applied in the field of medicine to achieve the effects of improving drying rate, uniform content and improving compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
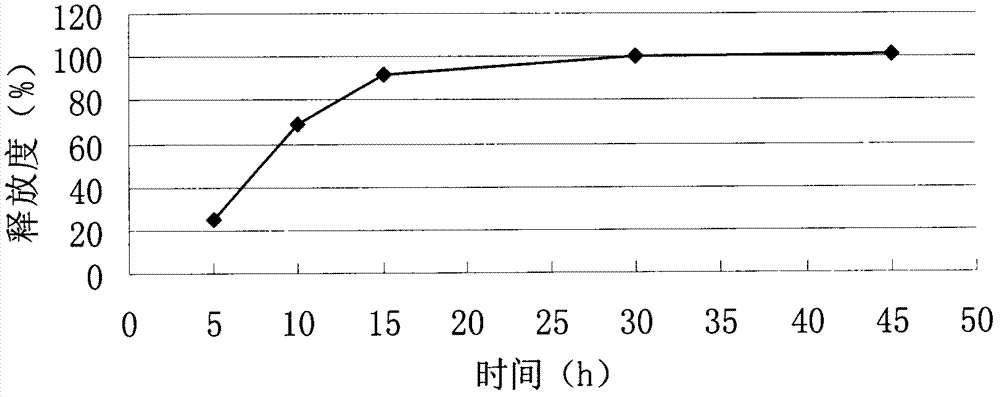
[0056] Example 1: Tablet formulation (50 mg tablet)
[0057] 1.1 The formula of imatinib mesylate tablet core in the present embodiment is as shown in Table 1.
[0058] The imatinib mesylate tablet core formula of table 1 embodiment 1 (making 10000 altogether)
[0059]
[0060] 1.2 Prepare the imatinib mesylate tablet core of the above-mentioned formula, carry out according to the following steps:
[0061] 1.2.1 The raw material is air-pulverized, and the particle size is less than 20um.
[0062] 1.2.2 Mix imatinib mesylate, microcrystalline cellulose and sodium carboxymethyl starch evenly, add polyvinylpyrrolidone K30 solution with a concentration of 12% by mass percentage, carry out rapid stirring and granulation, and obtain soft material Fluidized bed drying under the condition of wind temperature 65 ℃.
[0063] 1.2.3 After drying, add sodium carboxymethyl starch, micropowder silica gel and magnesium stearate, mix well, and press into tablets. Among them, the diamete...
Embodiment 2
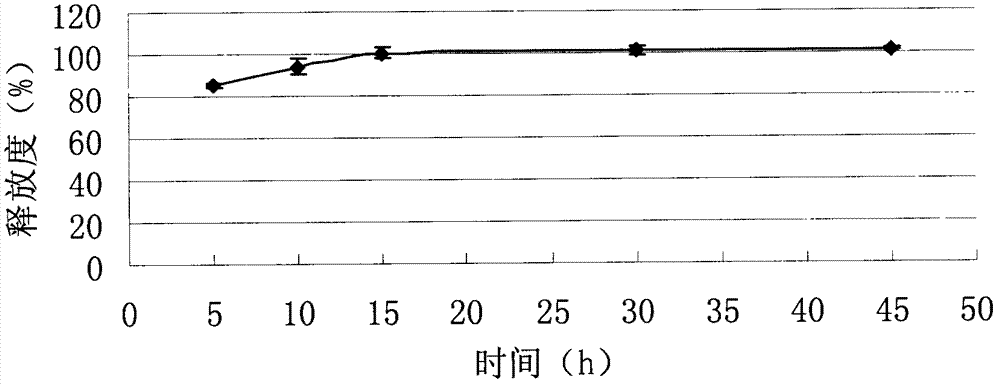
[0069] Example 2: Tablet formulation (100 mg tablet)
[0070] 2.1 The formula of imatinib mesylate tablet core in the present embodiment is as shown in Table 3.
[0071] The imatinib mesylate tablet core formula of table 3 embodiment 2 (making 5000 altogether)
[0072]
[0073] 2.2 To prepare the imatinib mesylate tablet core of the above prescription, proceed according to the following steps:
[0074] 2.2.1 Raw materials are air-pulverized, particle size <20um.
[0075] 2.2.2 Add imatinib mesylate, microcrystalline cellulose and croscarmellose sodium into the fluidized bed equipped with top spray equipment as the base, and hydroxypropyl cellulose is dissolved in water to form a mass percentage The mixed liquid with a concentration of 11.8% is sprayed onto the substrate in a fluidized state to form granules. The material temperature is about 35°C for spraying and granulation, and the inlet air temperature is 70°C for drying.
[0076] 2.2.3 After drying, add microcrystall...
Embodiment 3
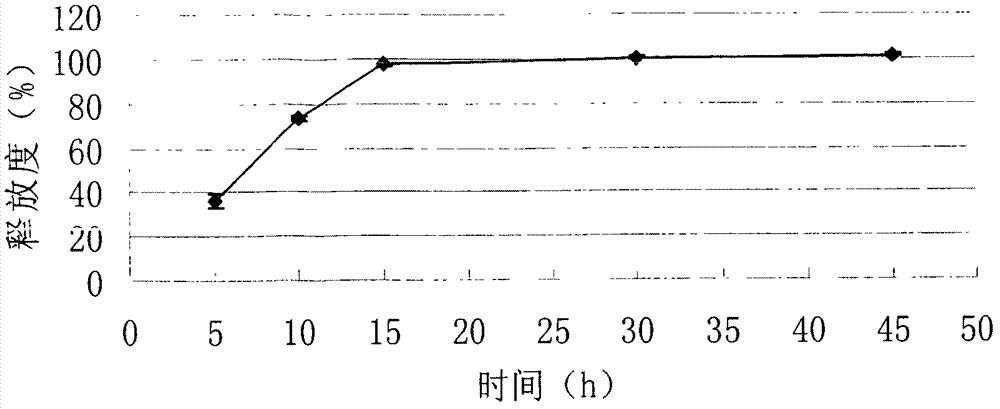
[0081] Example 3: Tablet formulation (100 mg tablet)
[0082] 3.1 The formula of imatinib mesylate tablet core in the present embodiment is as shown in Table 5.
[0083] The imatinib mesylate tablet core formula of table 5 embodiment 3 (making 5000 altogether)
[0084]
[0085] 3.2 To prepare the imatinib mesylate tablet core of the above prescription, follow the steps below:
[0086] 3.2.1 Raw materials are air-pulverized, particle size <20um.
[0087] 3.2.2 Mix imatinib mesylate, microcrystalline cellulose and cross-linked polyvinylpyrrolidone evenly, add a hydroxypropyl methylcellulose solution with a mass percentage concentration of 11.1% for rapid stirring and granulation, and obtain a soft material Fluidized bed drying at an inlet air temperature of about 65°C.
[0088] 3.2.3 After drying, add microcrystalline cellulose, cross-linked polyvinylpyrrolidone, micropowder silica gel and magnesium stearate, mix well, and press into tablets. Wherein, the diameter of the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com