Proline sulfonamide derivatives as orexin receptor antagonists
A technology of amide and appetite, applied in the field of formula-proline sulfonamide compound, preventing or treating diseases or diseases related to the orexin system, and can solve problems such as the use of undisclosed compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
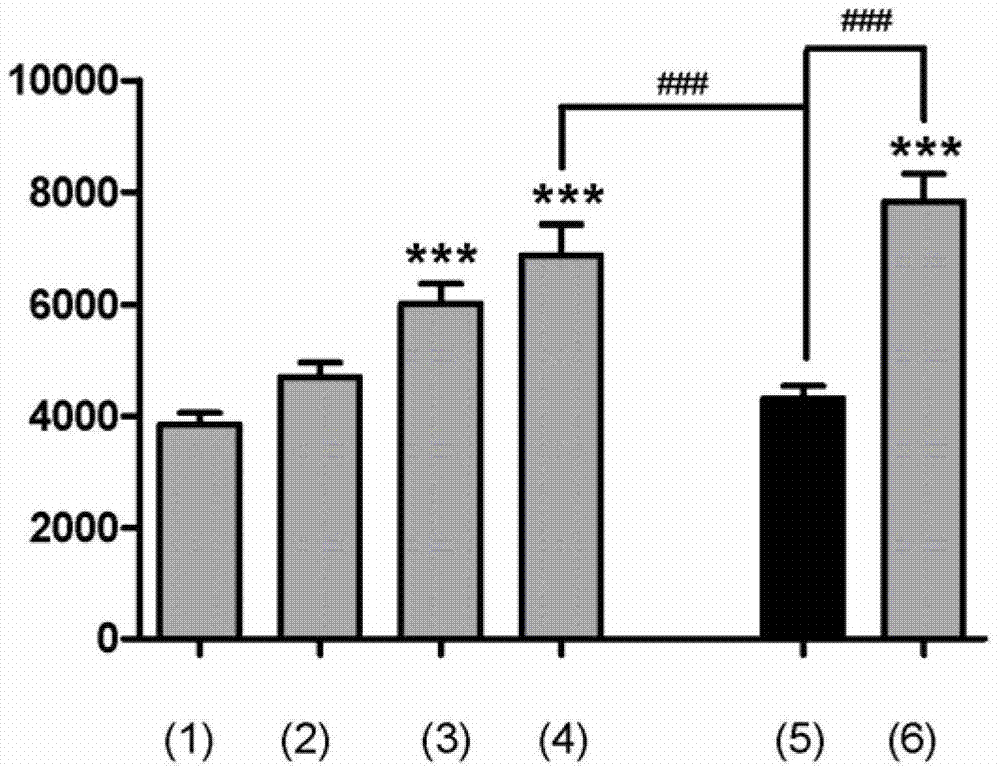
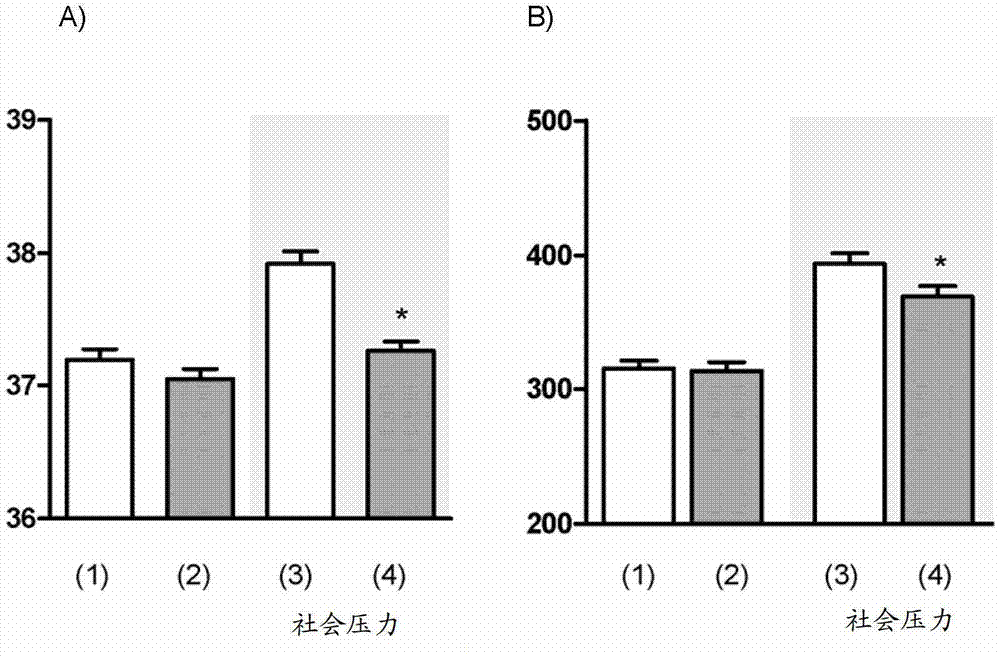
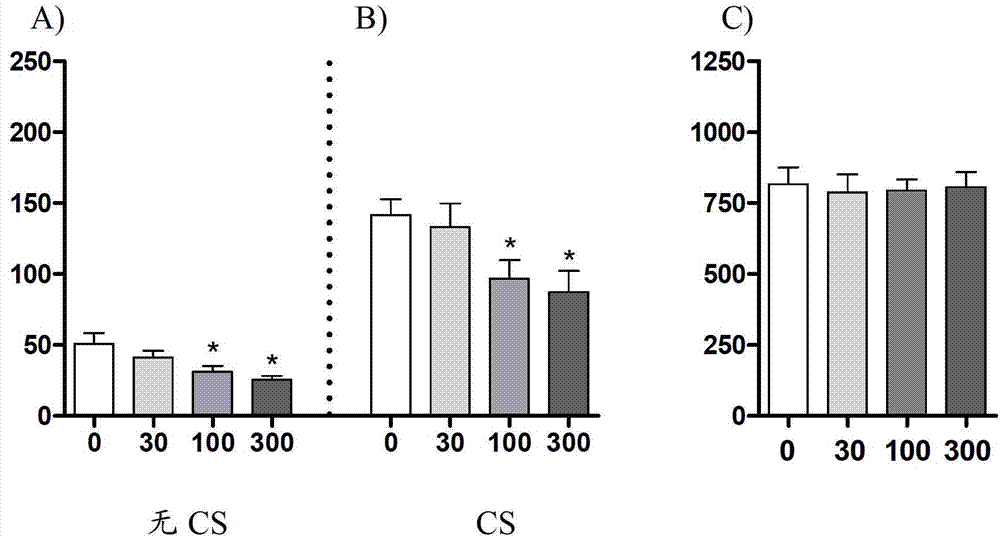
Image
Examples
preparation example Construction
[0233] Preparation of compounds of formula (I) can alternatively be carried out as depicted in Scheme 2, starting with Boc-L-proline (7), which is dissolved in a solvent such as DCM or MeCN, followed by addition of base (such as NEt 3 or DIPEA or N-methylmorpholine), activator ethyl chloroformate and aniline derivatives 5. Pure Boc-protected L-prolineaniline 8 can be isolated after aqueous work-up and chromatographic purification. Compound 9 was obtained by treating compound 8 with 4M HCl in dioxane (1,4-dioxane) at room temperature. Final compound 10 was prepared by reacting precursor 9 with sulfonyl chloride 2 in a solvent such as MeCN or DCM in the presence of a base such as DIPEA or N-methylmorpholine at room temperature, followed by aqueous work-up and purification.
[0234] Experimental part:
[0235] Abbreviations (as used here and in the specification above):
[0236] aq. Aqueous solution / water-based
[0237] BSA bovine serum albumin
[0238] CC silica gel column...
example
[0285] The example compounds listed in Table 1 below have been prepared according to Method A or B above using appropriate commercially available amine and sulfonyl chloride derivatives as starting materials.
[0286] To further characterize these compounds, antagonistic activity at two orexin receptors has been determined for each of the example compounds using the following procedure:
[0287] In Vitro Assay: Intracellular Calcium Measurement:
[0288] Chinese hamster ovary (CHO) cells expressing human orexin-1 receptor and human orexin-2 receptor were treated with 300 μg / ml G418, 100 U / ml penicillin, 100 μg / ml streptomycin and 10% heat-inactivated fetal calf serum (FCS) medium (Ham F-12 and L-glutamine) growth. Cells were seeded at 20,000 cells / well in 384-well black clear bottom sterile dishes (Greiner). at 37°C in 5% CO 2 Inoculated plates were incubated overnight in .
[0289] Human orexin-A as an agonist was prepared as a 1 mM stock solution in MeOH:water (1:1), dil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com