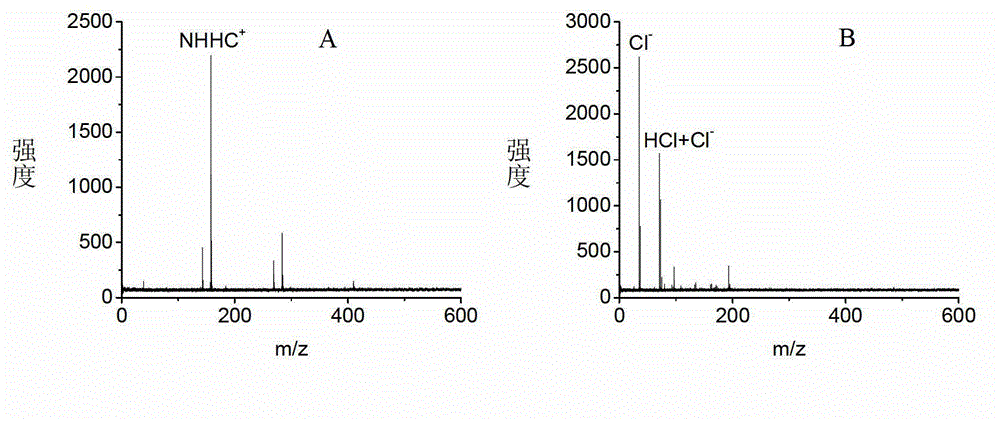
Application of naphthylhydrazine inorganic acid salt or Naphthylhydrazine organic acid salt as matrix in MALDI MS (matrix-assisted laser desorption/ionization mass spectrometry)
A technology of matrix-assisted laser and inorganic acid salts, which is applied in the field of mass spectrometry detection, can solve the problems of long time consumption, reduced analysis effect, and poor reusable effect, and achieve the effect of good sample compatibility and reduced requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
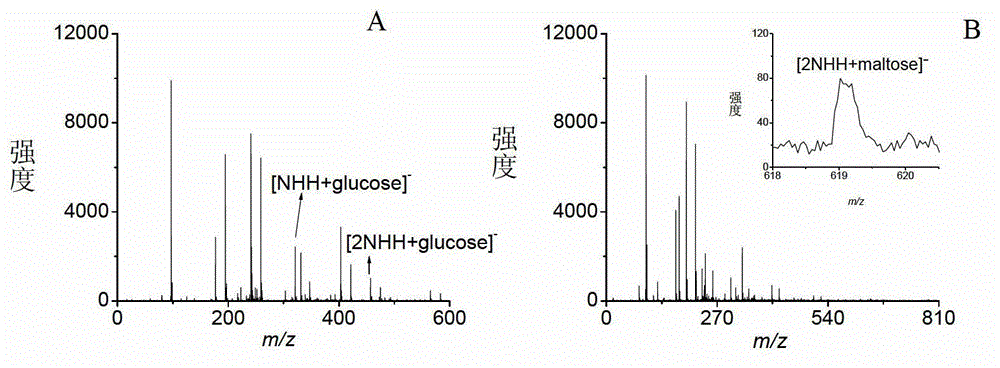
[0033] Embodiment 1, the analysis of oligosaccharides
[0034] Take various prepared sample solutions and matrix solutions and mix them uniformly at a volume ratio of 1:1 (the molar ratios of 1-naphthylhydrazine hydrochloride to glucose, sucrose, maltose, and maltoheptaose are 5:1, 5:1, and 1.5: 1 and 1.5:1), then add 1 μL of the mixed sample on the MALDI target plate, dry in the air and enter the mass spectrometry analysis.
[0035] Among them, glucose, sucrose, maltose and maltoheptaose were all dissolved in pure water, the concentrations were 3mmol / L, 3mmol / L, 10mmol / L and 10mmol / L in turn, and the matrix 1-naphthylhydrazine hydrochloride was dissolved in the volume ratio of 1:1 Prepare a 3 mg / mL solution in acetonitrile / water solution.
[0036]The mass spectrometry conditions are: voltage: accelerating voltage: 19.000kV; delayed extraction voltage: 14.920kV; reflector voltage: 20.000kV; lens voltage: 7.000kV; frequency: 1.000Hz; laser energy: 75~80%; accumulation times: 8...
Embodiment 2
[0038] Embodiment 2, analysis of glucose content in serum
[0039] Mix 10 μL human serum with 30 μL acetonitrile, mix well, centrifuge, take 10 μL supernatant and mix with 10 μL matrix 1-naphthylhydrazine hydrochloride solution, and finally take 1 μL of the mixed solution and add it to the MALDI target plate, dry it in the air and enter the mass spectrometry analysis .
[0040] When using the standard addition method to quantitatively detect the glucose concentration, prepare isotope-labeled D-glucose-1,2- 13 C 2 aqueous solution, and glucose aqueous solutions with molar concentrations of 0, 1, 2, 3, 5 and 9 mmol / L respectively. Take the prepared deproteinized serum supernatant and mix it with 3 mg / mL matrix aqueous solution, isotope-labeled glucose aqueous solution and glucose solutions of different concentrations at a volume ratio of 1:1:1:1, and take 1 μL of the mixed solution for MALDI-TOF mass spectrometry analyze.
[0041] The mass spectrometry conditions are: voltag...
Embodiment 3
[0044] Example 3, Analysis of Homogentisic Acid Content in Urine
[0045] Take fresh urine to prepare 5 mg / mL homogentisic acid solution, take out 1 μL of the mixed solution and add it to the MALDI target plate, dry it in the air, and then enter the mass spectrometry analysis.
[0046] When drawing a standard curve to quantitatively detect homogentisic acid in urine of known concentration, prepare isotope-labeled homogentisic acid with a molar concentration of 3 mg / mL- 13 C 6 The urine sample solution was mixed with the urine sample solution of 0, 1, 2, 3, 4 and 5 mg / mL of molar concentration respectively and 3 mg / mL matrix aqueous solution in equal volumes (wherein 1-naphthylhydrazine hydrochloride The molar ratio to homogentisic acid is about 3:5), take 1 μL of the mixed solution for MALDI-TOF mass spectrometry analysis.
[0047] The mass spectrometry conditions are: voltage: accelerating voltage: 19.000kV; delayed extraction voltage: 14.920kV; reflector voltage: 20.000kV;...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com