Hydrophobically modified polyethylenimines and their use as protein carriers
A polyethyleneimine and protein technology, applied in the biological field, can solve the problems of small molecular weight, inability to form nanoparticles, weak protein binding ability, etc., to reduce cytotoxicity, improve the effect of antigen cross-presentation, and improve the effect of immunotherapy. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The synthetic schematic diagram of the hydrophobically modified polyethyleneimine of this embodiment is as figure 1 As shown, the specific steps are as follows:
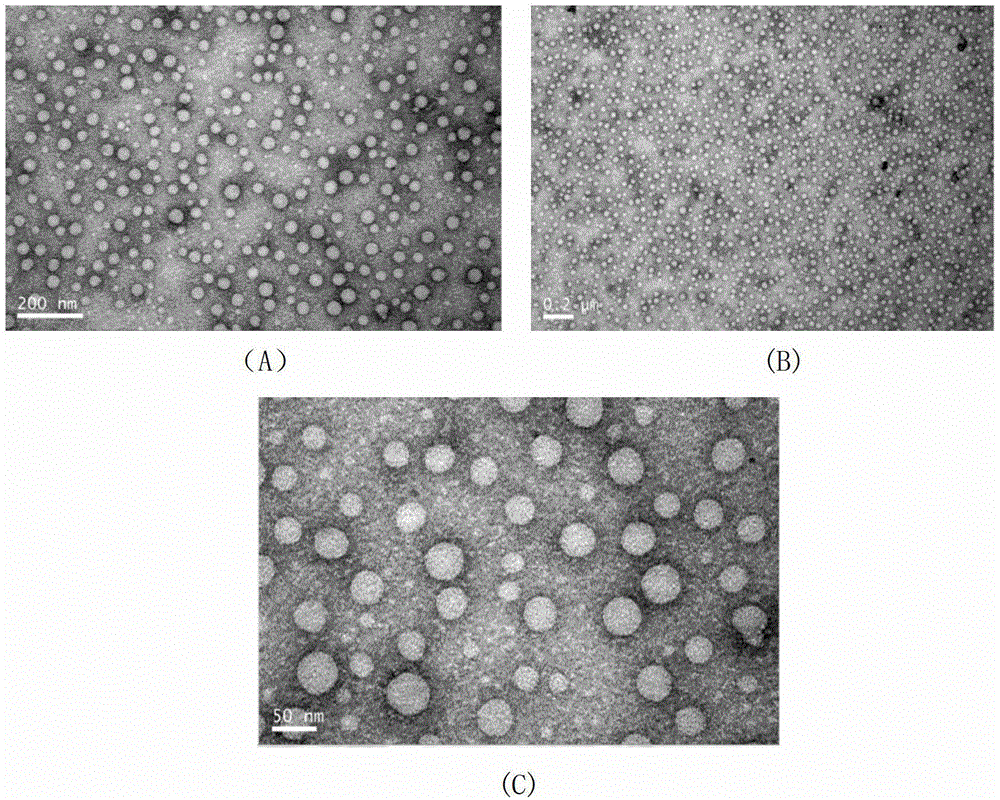
[0037] First, dissolve polyethyleneimine PEI-423Da in a dehydrated and dried organic solvent (dichloromethane, chloroform, tetrahydrofuran, methanol, ethanol, propanol or acetonitrile, dichloromethane is used in this example), and add Two-fold molar ratio of 1-substituted chain lipid CH 3 -(CH 2 ) 11 -Br, the mixture is reacted at 20-100°C for 0.5-50h and then distilled under reduced pressure to remove dichloromethane, and then precipitated and washed with ether, and then separated and purified to obtain the structural formula The final product represented is the lipid hydrophobic modified polyethyleneimine 2C1 2 -PEI-423Da(C12PEI 423 ). 1 HNMR (CDCl 3 , 300MHz) 4.3 (wide peak, NH), 3.2-2.2 (wide peak, N-CH 2 CH 2 -N, 34H), 1.8-1.15 (broad peak, fatty chain CH 2 , 40H), 0.94-0.72 (triplet, terminal CH 3 , 6H).
[0...
Embodiment 2
[0046] The material involved in this example is the same as that of Example 1. The synthesis process of hydrophobically modified polyethyleneimine and the composite method of nanoparticles are also the same as that of Example 1. The difference is that the chain lipid during synthesis is CH 3 -(CH 2 ) 7 -Br, the final product prepared is the lipid hydrophobically modified polyethyleneimine 2C8-PEI-423Da (C8PEI 423 ) Such as structural formula Shown. 1 HNMR (CDCl 3 , 300MHz) 4.3 (wide peak, NH), 3.2-2.2 (wide peak, N-CH 2 CH 2 -N, 34H), 1.8-1.15 (broad peak, fatty chain CH 2 , 12H), 0.94-0.72 (triplet, terminal CH 3 , 6H).
[0047] Prepare C in the same way as in Example 1. 8 PEI 423 / OVA complex. The measured particle size is 205.8nm, and the Zeta potential is -12.8mV. Antigen cross-presentation effect such as Figure 4 Shown by Figure 4 It can be seen that: the prepared C 8 PEI 423 / OVA composite nanoparticles have a significant enhancement effect on antigen cross-presentation i...
Embodiment 3
[0049] The material involved in this example is the same as that of Example 1. The synthesis process of hydrophobically modified polyethyleneimine and the composite method of nanoparticles are also the same as that of Example 1. The difference is that the chain lipid during synthesis is CH 3 ——(CH 2 ) 19 -CI, the final product prepared is the lipid hydrophobic modified polyethyleneimine 2C20-PEI-423Da (C20PEI 423 ), such as structural formula Shown. 1 HNMR (CDCl 3 , 300MHz) 4.3 (wide peak, NH), 3.2-2.2 (wide peak, N-CH 2 CH 2 -N, 34H), 1.8-1.15 (broad peak, fatty chain CH 2 , 72H), 0.94-0.72 (triplet peak, terminal CH 3 , 6H).
[0050] Prepare C in the same way as in Example 1. 20 PEI 423 / OVA complex. The measured particle size is 275.8nm, and the Zeta potential is -9.0mV. Antigen cross-presentation effect such as Figure 5 Shown by Figure 5 It can be seen that: the prepared C 20 PEI 423 / OVA composite nanoparticles have a significant enhancement effect on antigen cross-present...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com