Rofecoxib-like derivative, prepared organic fluorescent dye skeleton and application
A technology of rofecoxib and its derivatives, which is applied in the field of biological imaging and the field of organic fluorescent dye skeletons, can solve the problems of inability to meet the needs of analysis and detection, different photophysical and photochemical properties, and achieve improved photophysical and photochemical properties. Photochemical performance, improvement of photophysical and photochemical performance, effect of simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
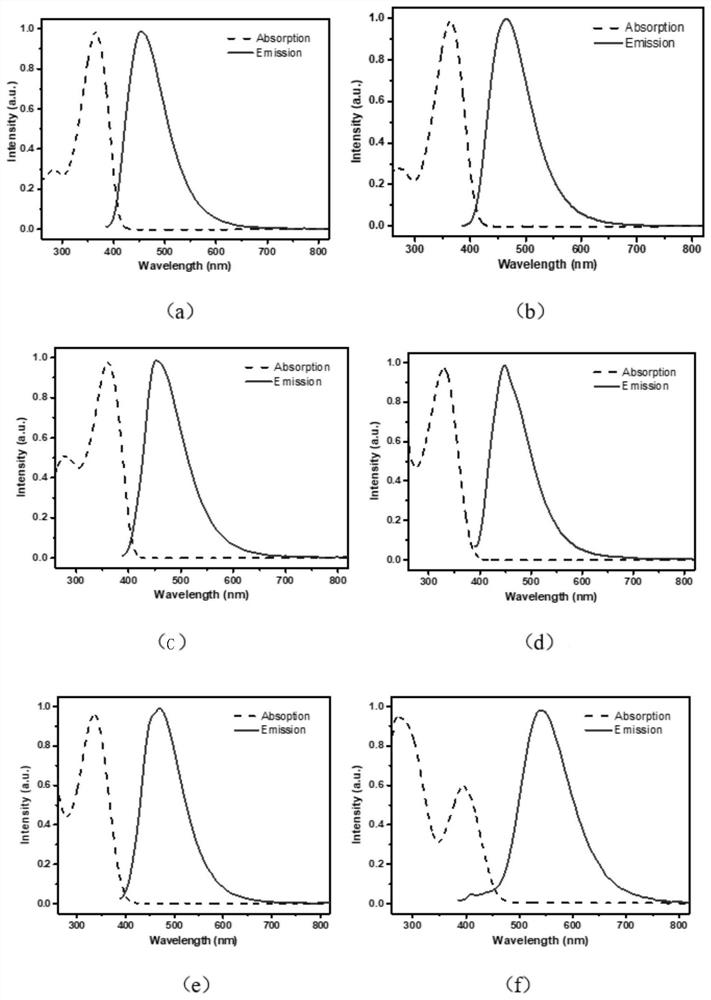
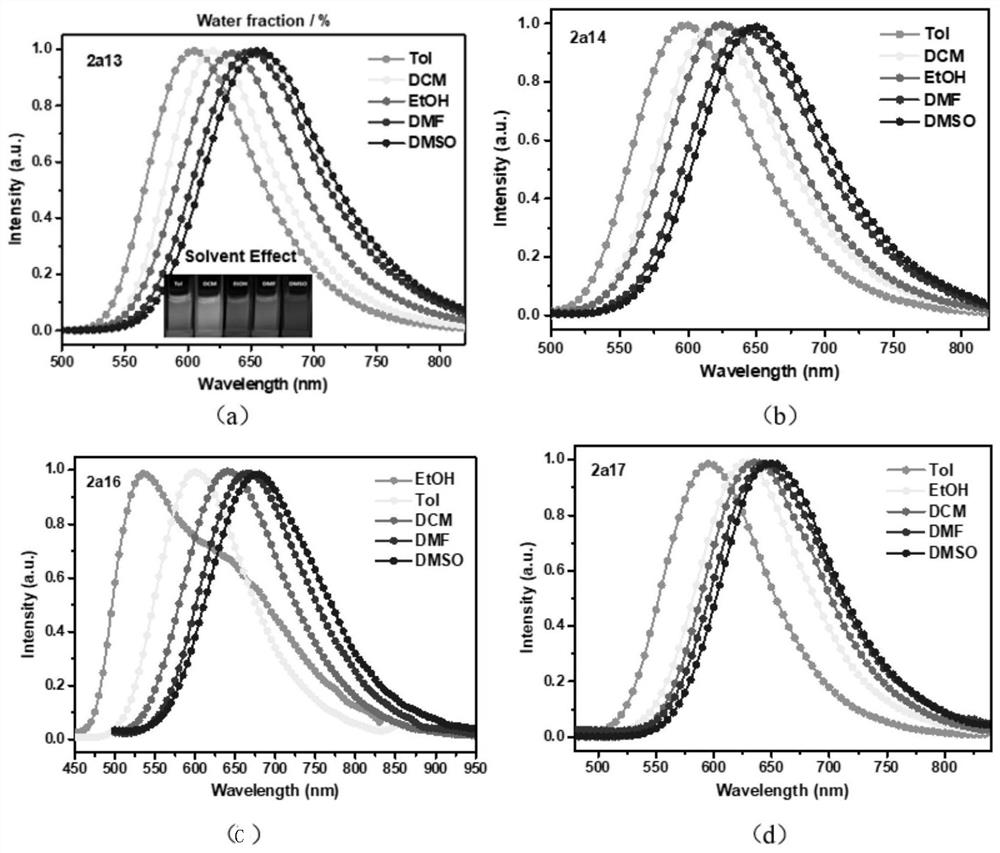
Image
Examples
Embodiment 1
[0056] Example 1: Compound 2a1((5Z)-5-(4-cyanobenzylidene)-4-(4-(methylsulfonyl)phenyl)-3-phenylfuran-2(5H)-one )
[0057]
[0058] Add 0.12 g (0.0014 mol) of piperidine dropwise to 0.4 g (0.0013 mol) of compound 1a or 1b and 0.0026 mol of aromatic aldehyde in methanol mixed solution, react at room temperature and stir in a dark room for 12 hours, and the reaction solution reacts after detection After completion, after cooling and suction filtration, and washing with methanol, 0.28 g (89%) of the product was obtained as light yellow solid powder.
[0059] The nuclear magnetic data of gained compound is: 1 H-NMR (400MHz, DMSO-d 6 )δ8.04(d, J=8.5Hz, 2H), 7.96(d, J=8.6Hz, 2H), 7.88(d, J=8.6Hz, 2H), 7.67(d, J=8.5Hz, 2H) ,7.35–7.31(m,5H),6.16(s,1H),3.28(s,3H). 13 C-NMR (101MHz, DMSO-d 6 )δ167.77,149.99,149.06,142.31,138.10,135.11,133.20,131.37,130.90,129.95,129.93,129.72,129.07,128.95,128.19,127.59, 119.23,111.32,111.12,43.78.HR-MS(ESI):calcd for C 25 h 17 NNaO 4 S:450....
Embodiment 2
[0060] Example 2: Compound 2a2 ((5Z)-5-(benzylidene)-4-(4-(methylsulfonyl)phenyl)-3-phenylfuran-2(5H)-one)
[0061]
[0062] The preparation steps of compound 2a2 in this example are the same as those in Example 1, using compound 1a as the starting material to synthesize a yellow powder, and the calculated product yield is 86%.
[0063] The nuclear magnetic data of gained compound is: 1 H-NMR (400MHz, DMSO-d 6 )δ8.04(d, J=8.7Hz, 2H), 7.79(d, J=7.4Hz, 2H), 7.67(d, J=8.8Hz, 2H), 7.47–7.39(m, 3H), 7.38– 7.27(m,2H), 6.06(s,1H),3.28(s,3H). 13 C-NMR (101MHz, DMSO-d 6 )δ168.12, 149.44, 147.98, 142.21, 135.46, 133.47, 131.02, 130.88, 129.87, 129.65, 129.51, 129.22, 129.02, 128.15, 126.28, 113.39, 43.79.HR-MS 24 h 18 NaO 4 S:425.0823([M+Na] + ), found: 425.0817. It can be seen that the structure of the product is correct.
Embodiment 3
[0064] Example 3: 2a3((5Z)-5-(4-methylsulfonylbenzylidene)-4-(4-(methylsulfonyl)phenyl)-3-phenylfuran-2(5H)-one )
[0065]
[0066] The preparation steps of compound 2a3 in this example are the same as in Example 1, using compound 1a as the starting material to synthesize a yellow powder, and the calculated product yield is 88%.
[0067] The nuclear magnetic data of gained compound is: 1 H-NMR (400MHz, DMSO-d 6 )δ8.09–8.00(m,4H),7.96 (d,J=8.5Hz,2H),7.68(d,J=8.5Hz,2H),7.39–7.25(m,5H),6.19(s,1H ),3.28(s,3H), 3.22(s,3H). 13 C-NMR (101MHz, DMSO-d 6 )δ167.86,149.90,149.12,142.30,140.78,138.35,135.14,131.42,130.92,129.73,129.70,129.11,129.08,128.97,128.19,127.99,127.97,127.55, 111.10,43.79.HR-MS(ESI):calcd for C 25 h 20 NaO 6 S 2 :503.0599([M+Na] + ), found: 503.0588. It can be seen that the structure of the product is correct.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com