Double-ligand collecting agent and preparation and application thereof
A collector, dithiourea technology, applied in solid separation, organic chemistry, flotation, etc., can solve the problems of insufficient mineral surface action, inability to disperse well, and high melting point of the collector, and improve the comprehensive recovery of metals. efficiency, reducing the dosage of inhibitors, and the effect of strong harvesting ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
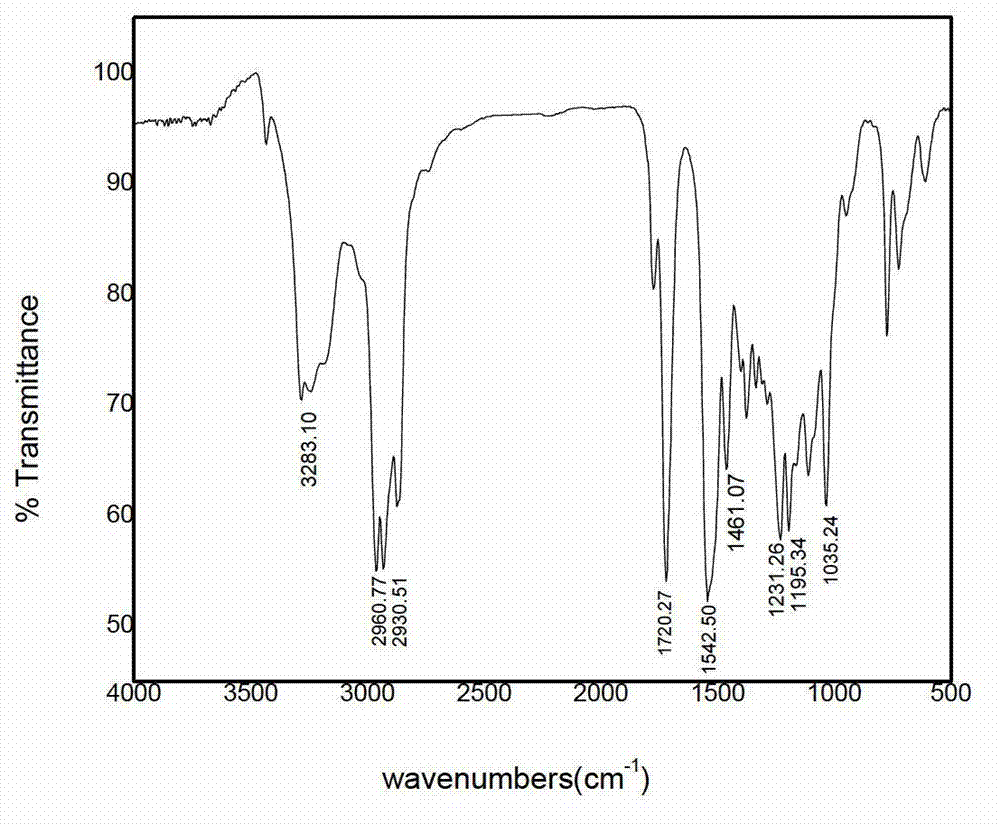
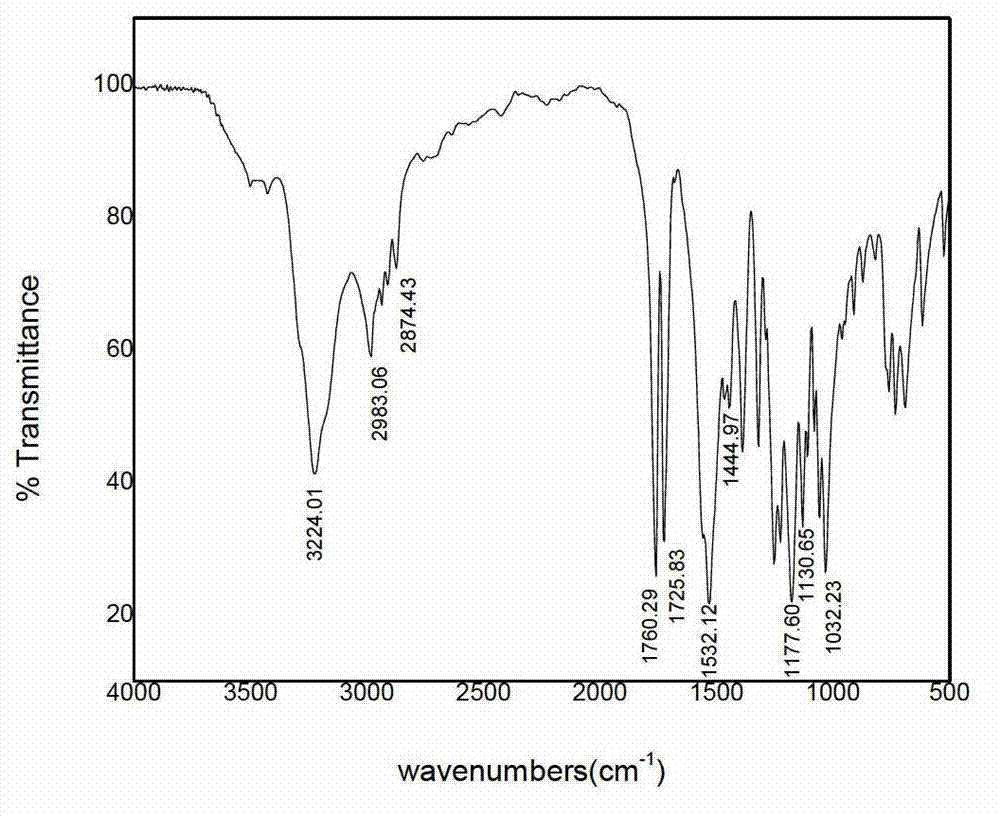
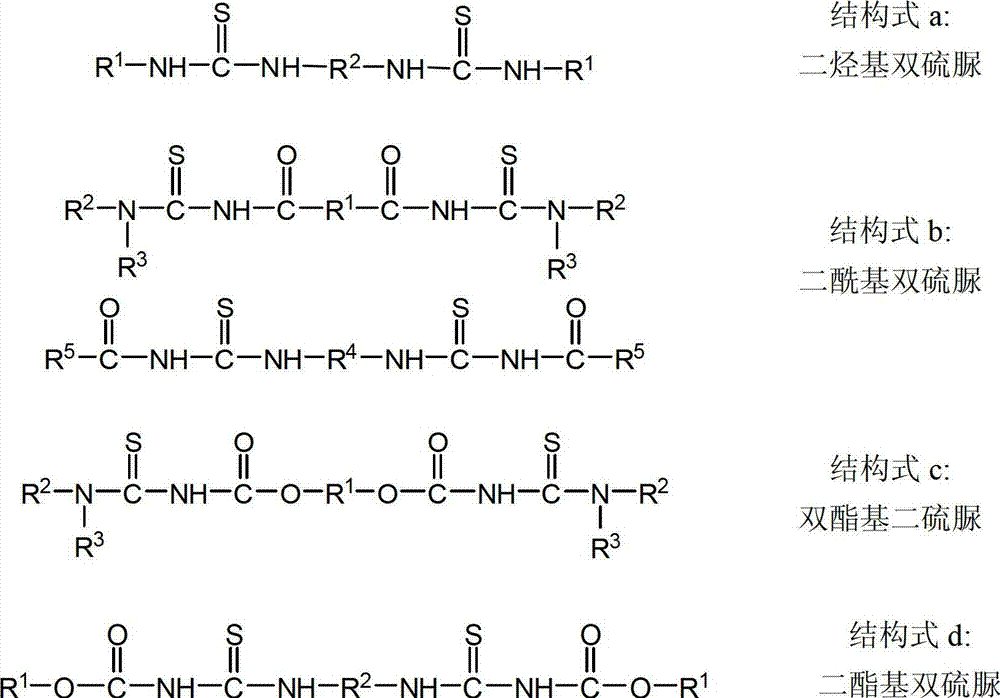
Image
Examples
Embodiment 1
[0030] Embodiment 1N, the preparation of N'-diisooctyloxycarbonyl-N ", N "'-isopropylidene dithiourea
[0031] In an aqueous solution containing 431 parts of N-isooctyloxycarbonyl isothiocyanate, slowly drop into an aqueous solution containing 230 parts of polyetheramine D-230 with a concentration of 30%, and stir and react at 0°C to 10°C for 2 hours. Then react at room temperature for 30 minutes to obtain 647 parts of N,N'-diisooctyloxycarbonyl-N",N"'-isopropylidene dithiourea with a yield of 98%.
Embodiment 2
[0032] Example 2N, N'-diethoxycarbonyl-N", the preparation of O-ethylene ether group thiourethane thiourea complex base compound
[0033] In the n-hexane solution containing 262 parts of N-ethoxycarbonyl isothiocyanate, slowly drop 105 parts of diglycolamine, stir and react at 0°C to 15°C for 2 hours, then raise the temperature to 55°C for 2 hours, 356 parts of N,N'-diethoxycarbonyl-N",O-ethylene ether thiocarbamate thiourea were obtained with a yield of 97%.
Embodiment 3
[0034] Embodiment 3N, the preparation of N'-dibutyryl-N ", N "'-ethylene ether group dithiourea
[0035] In the n-hexane solution containing 259 parts of N-butyryl isothiocyanate, slowly drop into the n-hexane solution containing 104 parts of 2,2'-oxybis(ethylamine), and stir the reaction at 0 ° C ~ 25 ° C 2 hours, and then raised the temperature to 45°C for 2 hours to obtain 348 parts of N,N'-dibutyryl-N",N"'-ethylene ether dithiourea with a yield of 96%.
[0036] (2) Application of ether-based dithiourea or thiourethane-thiourea complex compound in metal ore flotation
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com