Synthesis method for thiophene ring/furan ring-heteroaromatic ring structure
A compound and alkyl technology, applied in the field of chemistry, can solve problems such as poor compatibility of functional groups and lengthy reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
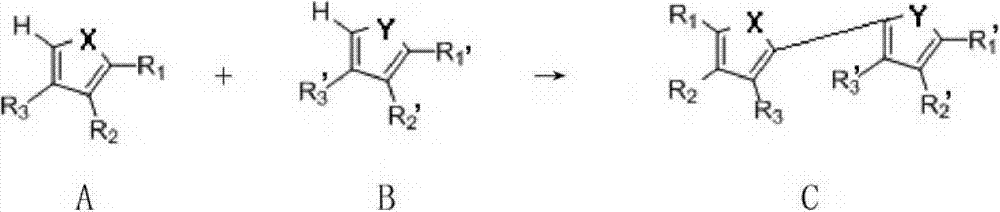
[0049]The present invention provides a method for preparing a thiophene ring / furan ring and its derivatives-heteroaromatic ring building blocks. Preferably, the method comprises the steps of:
[0050] In an inert solvent, at a certain temperature (such as 40°C-140°C; preferably 60-100°C), using palladium salt as a catalyst, in the presence of an oxidizing agent, the compound of formula A (ie thiophene ring or furan ring or Its derivative) reacts with the compound of formula B (ie heteroaromatic ring compound) for a period of time (such as 1-20 hours or 5-10 hours, etc.), thereby forming the compound of formula C (ie thiophene ring / furan ring and its derivatives -heteroaromatic ring building blocks);
[0051]
[0052] In various forms, R 1 , R 2 , R 3 , R' 1 , R' 2 , R' 3 , X, Y are defined as mentioned above.
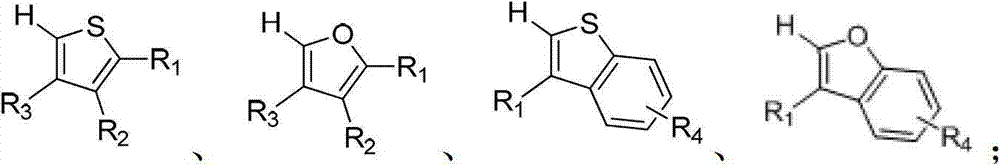
[0053] Wherein, the compound of formula A is preferably a compound selected from the following group:
[0054]
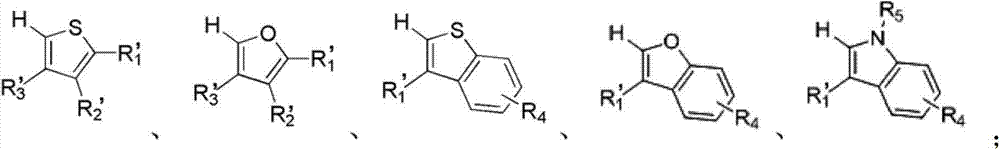
[0055] In various forms, R 1 , R 2 , R ...
Embodiment 1
[0075]
[0076] Into a 25 mL reaction tube, add 6.7 mg (10 mol%) of Pd(OAc) 2 , AgOAc (0.9mmol), 2-N,N-dimethylformamidothiophene (0.3mmol, 1 equiv), nitrogen replacement three times and then add 2mL dimethylformamide (DMF), inject 87μL (0.45mmol)2 -Bromo-3-hexylthiophene, 30% yield after stirring at 80 °C for 8 hours.
Embodiment 2
[0078]
[0079] Into a 25 mL reaction tube, add 6.7 mg (10 mol%) of Pd(OAc) 2 , AgOAc (0.9mmol), 2-N,N-dimethylformamidothiophene (0.3mmol, 1 eq), nitrogen replacement three times, adding 2mL dimethylsulfoxide (DMSO), injecting 87μL (0.45mmol)2 -Bromo-3-hexylthiophene, after stirring at 80°C for 8 hours, the target compound was obtained by column separation with a yield of 28%.
[0080] 1 H NMR (300MHz, CDCl 3 )δ7.24(d,J=3.9Hz,1H),7.00(d,J=3.9Hz,1H),6.92(s,1H),3.19(s,6H),2.53(t,J=7.8Hz, 2H),1.57(m,2H),1.32(m,6H),0.89(t,J=6.6Hz,3H). 13 C NMR (75.4MHz, CDCl 3 )δ163.7, 143.1, 140.2, 136.4, 135.7, 130.0, 125.4, 122.9, 108.8, 31.5, 29.5, 29.4, 28.8, 22.5, 14.0.m.p.54°C.IR (thin film method): v max 3068,1610cm -1 .MS(EI):m / z(%)401(M + ),399(M + ), 274(100), 243, 171.HRMS: Calculated for (theoretical value): C 17 h 22 NOS 2 Br: 399.0326; Found (measured value): 399.0331.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com