Method for preparing ethylene by ethanol dehydration
A technology for ethanol dehydration and ethylene, applied in chemical instruments and methods, hydrocarbon production from oxygen-containing organic compounds, molecular sieve catalysts, etc., can solve the problems of poor reaction stability, etc., and achieve the suppression of carbon deposition, smooth channels, and good The effect of technical effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
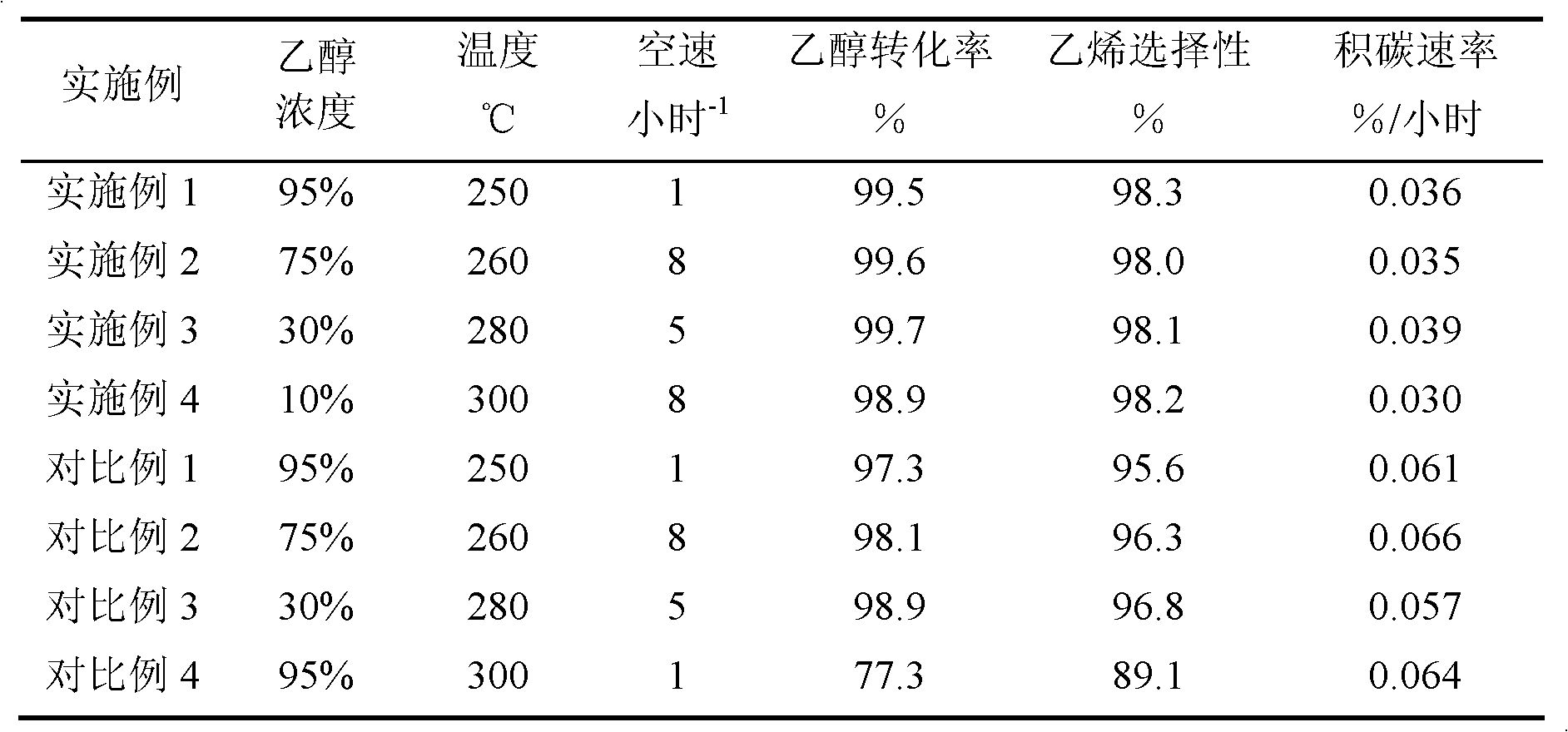
Examples
Embodiment 1
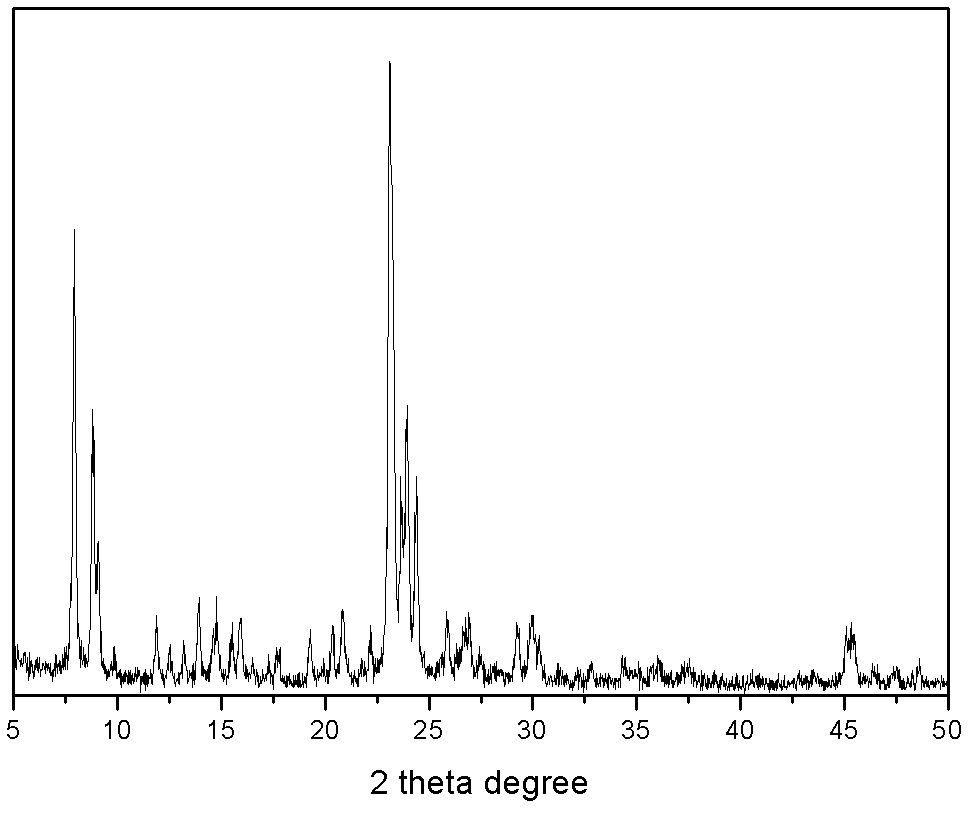
[0022] Weigh 7.4 grams of silica sol (SiO 2 40% by weight), then add sodium metaaluminate, 40% aqueous sodium hydroxide solution, so that the molar ratio is: 6 Na 2 O:Al 2 o 3 : 80 SiO 2 , and add water to mix, knead and extrude. After that, it was dried at 100° C. for 1 hour, and then pelletized. A mixture of 2 grams of tetrapropylammonium bromide and 10 grams of distilled water was added in advance in the reaction kettle, a stainless steel mesh was placed above the mixture, the formed molecular sieve was placed on the stainless steel mesh, and the reaction kettle was sealed. The reactor was subjected to gas-solid phase treatment at 150°C for 4 days. After the product was taken out, it was washed with water, dried at 120°C for 10 hours, and then baked at 550°C for 5 hours to remove the template agent. For the XRD characterization results of the sample, see figure 1 . Afterwards, the obtained material was exchanged three times with 10% by weight ammonium nitrate aqueous...
Embodiment 2
[0025]Weigh 7.4 grams of silica sol (SiO 2 Weight content 40%), add sodium metaaluminate, 40% sodium hydroxide aqueous solution again, make mol ratio be: 8 Na 2 O:Al 2 o 3 : 100 SiO 2 , and add water to mix, knead and extrude. Thereafter, it was dried at 120° C. for 1 hour, and then pelletized. Add a mixture of 2 grams of tetrapropylammonium hydroxide and 10 grams of distilled water in advance in the reaction kettle, place a stainless steel mesh above the mixture, place the formed molecular sieve on the stainless steel mesh, and seal the reaction kettle. The reactor was subjected to gas-solid phase treatment at 170°C for 3 days. After the product was taken out, it was washed with water, dried at 120°C for 10 hours, and then baked at 550°C for 5 hours to remove the template. Afterwards, the obtained material was exchanged three times with 10% by weight ammonium nitrate aqueous solution at 80° C., washed twice with water, dried at 120° C. for 10 hours, and calcined at 550°...
Embodiment 3
[0028] Weigh 7.4 grams of silica sol (SiO 2 40% by weight), then add sodium metaaluminate, 40% aqueous sodium hydroxide solution, so that the molar ratio is: 3.5 Na 2 O:Al 2 o 3 : 60 SiO 2 , and add water to mix, knead and extrude. After that, it was dried at 120° C. for 2 hours and cut into pellets. A mixture of 6 g of ethylenediamine and 10 g of distilled water was preliminarily added to the reaction kettle, a stainless steel mesh was placed above the mixture, the formed molecular sieve was placed on the stainless steel mesh, and the reaction kettle was sealed. The reactor was subjected to gas-solid phase treatment at 180°C for 2 days. After the product was taken out, it was washed with water, dried at 120°C for 10 hours, and then baked at 550°C for 5 hours to remove the template. Afterwards, the obtained material was exchanged three times with 10% by weight ammonium nitrate aqueous solution at 80° C., washed twice with water, dried at 120° C. for 10 hours, and calcine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com