Artificial cellulosome and the use of the same for enzymatic breakdown of resilient substrates
A technology of complexes and derivatives, applied in the direction of enzymes, enzymes, enzyme stabilization, etc., can solve the problems that separation cannot be easily separated, inefficient, and expensive for industrial development.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Example 1: Isolation of mutants that do not form cellulosomes
[0109] Mutants were isolated from mutagenized cultures of C. thermocellulosa (Figure 1). Six colonies with decreased or lost ability to form a clear color circle in the cellulose surrounding the colony were randomly selected. One of the C. thermocellum mutants, SM1, completely lost the ability to produce the scaffold protein CipA or active adhesion factors. Enzymes from wild type (with cellulosomes) and from mutant SM1 (without cellulosomes; free enzyme). Enzyme activity on barley β-glucan and CMC was about 8.5 and 1.0 U mg for the two strains, respectively -1 Protein (Table 1). In contrast, in the mutant SM1, the specific activity on crystalline cellulose dropped dramatically, up to 15-fold compared to the wild type.
[0110] Except for the complete disappearance of the CipA component (scaffold protein CipA), the mutant produced roughly the same amount of cellulosomal components as compared to the w...
Embodiment 2
[0113] Example 2: Reconstitution of cellulosomes
[0114] A: Preparation of Enzyme Components
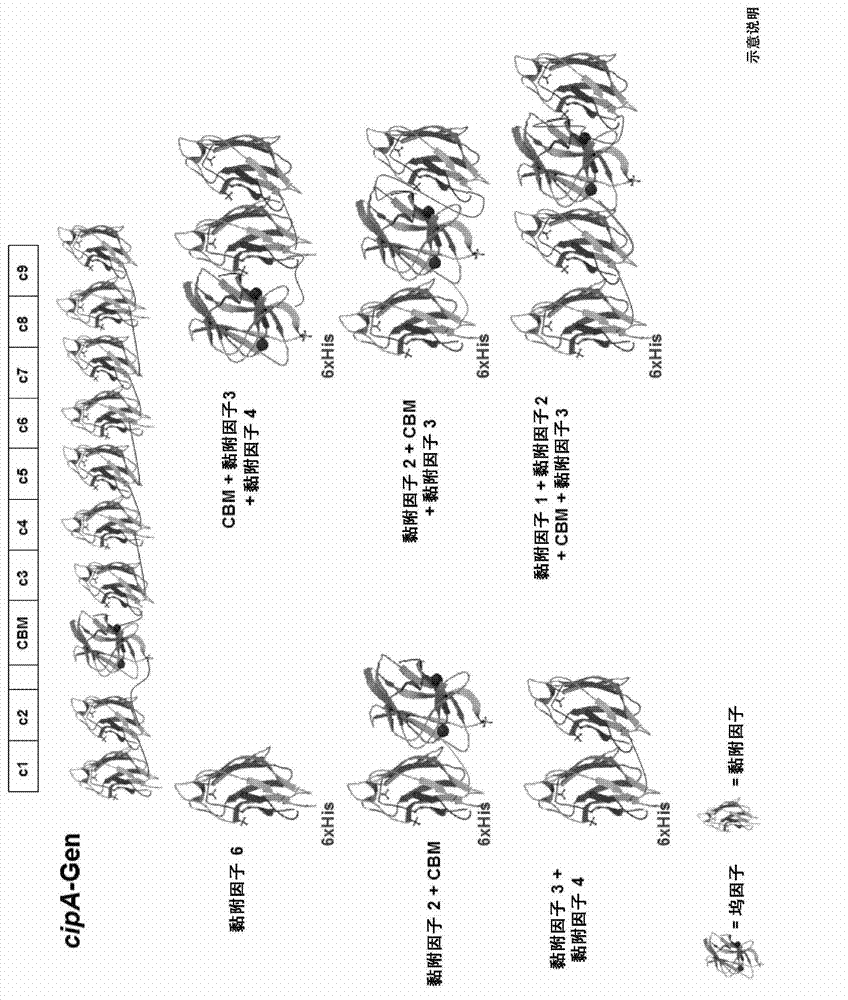
[0115] Mutant SM1 and mutant supernatant protein (SM901) were selected to reconstitute artificial cellulosomes. In addition, genes encoding cellulase components were cloned and characterized for their activity on biochemical parameters such as pH and temperature optima, as well as on different substrates. From previous data on cellulosome composition 11 Five of the most prominent enzyme components with cellulase activity were selected. Additionally, biochemical characterization of β-glucosidases from a large number of thermophilic saccharolytic bacteria. The β-glucosidase BglB from Thermotoga maritima was selected for its high thermostability and high activity on cellodextrins. This gene was fused to the dockerin module of the C. thermocellulase cellulase CelA. Determine optimal expression conditions. CelK-d1, CelR-d1, CelT-d1, CelE-d1, CelS-d1 and BglB-d1 are referred to ...
Embodiment 3
[0125] Example 3: Activity of artificial cellulosome complexes
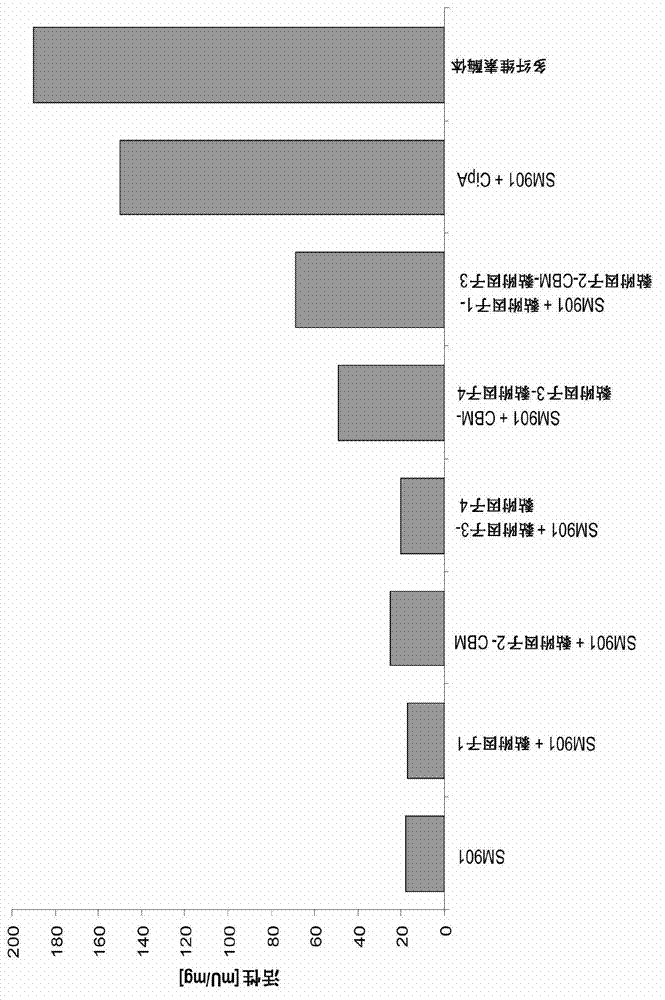
[0126] This mixture of complexes with and without CBM is now bound on the surface of polystyrene nanoparticles via optimized aliphatic linker molecules. This structure is schematically shown in FIG. 3 . Despite the inevitable loss of steric hindrance and freedom of enzymatic components due to the dense coverage of nanoparticles, the incorporation of various mini-scaffold protein complexes also resulted in increased activity during hydrolysis of crystalline cellulose (Fig. 3, 4). Additionally, the pH range of the enzyme is wider if the protein is bound to the particle (Figure 5). This also holds true for the temperature stability of the cellulase (Figure 6). Both results are important advantages for technical applications.
[0127] To test the feasibility of this approach, a portion of the SM901 component mixture was replaced with one or more recombinant cellulases. Although the SM901 component in the mixtur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com