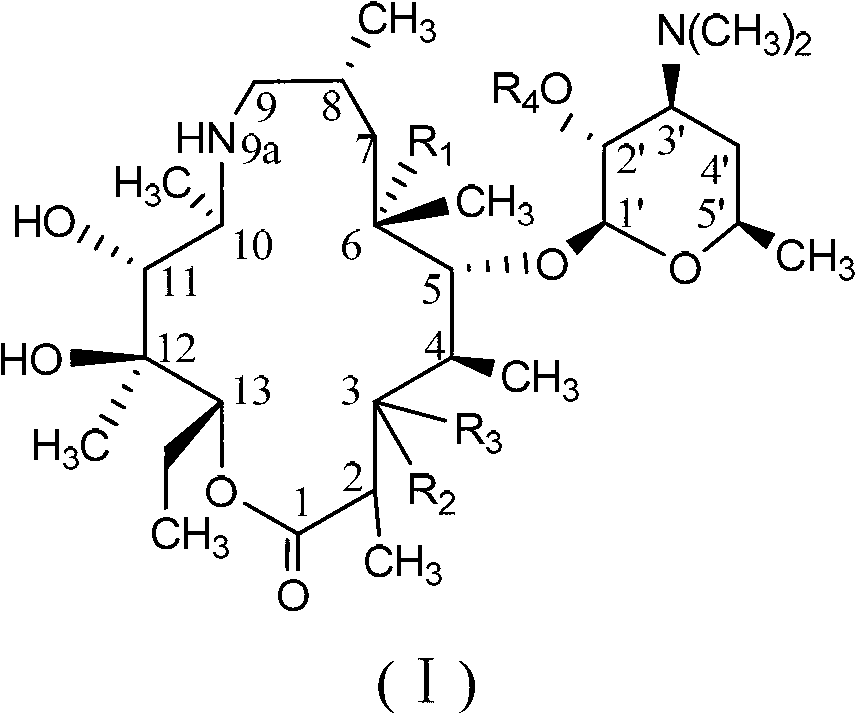
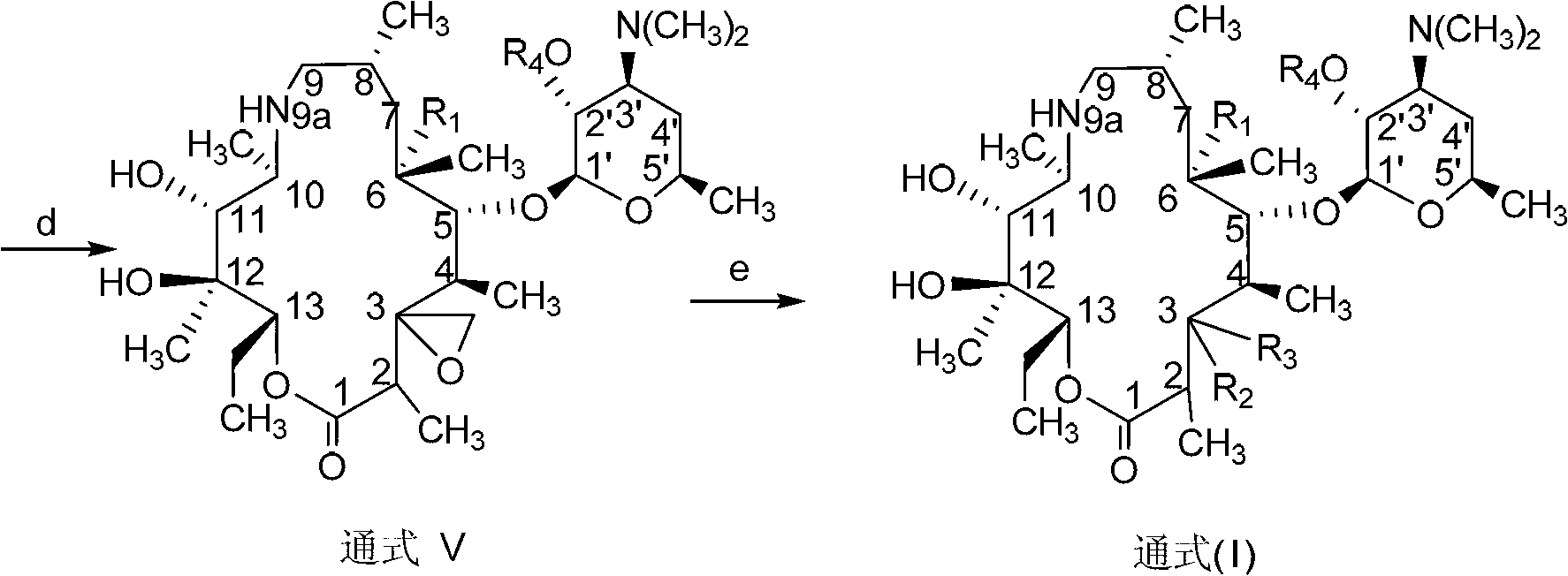
C-3 substituted-9-deoxidized-9A-aza-9A-high erythromycin A derivative
A technology of -13-, triple deoxygenation, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0267] The purpose of this example is to prepare the compound of the above-mentioned general formula 2, which is called: (2R, 3S, 4R, 5R, 8R, 10R, 11R, 12S, 13S, 14R)-13-[(2,6-dideoxy -3-C-methyl-3-O-methyl-α-L-hexapyranosyl-oxy]-2-ethyl-3,4,10-trihydroxy-3,5,8,10 , 12,14-Hexamethyl-11-[[3,4,6-trideoxy-3-(dimethylamino)-2-O-[(phenylmethoxy)carbonyl]-β-hexylpyridine Xylanosyl]oxy]-1-oxa-6-azacyclopentadecan-15-one.
[0268] Add 800mL of dichloromethane into a 2L three-neck reaction flask, cool to 0-5°C, stir, add 50g (0.068moL) of compound 1 (manufactured by Guobang Import and Export Company, batch number: 20091101), and stir until dissolved. Dissolve 28.8g (24mL; 0.1688moL) of benzyl chloroformate (Xinyi Huili Fine Chemical Co., Ltd., batch number: 20111008) into 60mL of dichloromethane, drop it in at constant pressure, and control the temperature at 0-5°C. After dropping, stir at this temperature for 1 h. After the reaction is completed, 50° C. is rotary evaporated under re...
Embodiment 2
[0274] The purpose of this example is to prepare the compound of the above general formula 3, which is named: (2R, 3S, 4R, 5R, 8R, 10R, 11R, 12S, 13S, 14R)-2-ethyl-3,4,10 , 13-tetrahydroxy-3,5,8,10,12,14-hexamethyl-11-[[3,4,6-trideoxy-3-(dimethylamino)-2-O[(benzene methoxy)carbonyl]-β-hexylopyranosyl]oxy]-1-oxa-6-azacyclopentadecane-15-one.
[0275] Add 1 L of 95% ethanol to a 5 L three-necked flask, heat and stir in a water bath at 50° C., weigh 300 g (0.345 moL) of compound 2 prepared by the method in Example 1, and then add 1 L of 95% ethanol to the reaction flask. Stir to dissolve; take 750mL (9.0moL) of concentrated hydrochloric acid in 1800mL water, stir and mix, and add to the reaction solution in batches. Reaction at 50°C for 22h. After the reaction is complete, cool to room temperature, extract with tert-butyl methyl ether (4000mL×3) in sequence to obtain the lower aqueous phase, then take 610g of sodium carbonate and dissolve it in 4000mL of water, stir to dissolve...
Embodiment 3
[0281] The purpose of this example is to prepare the compound of the above general formula 4, which is named: (2R, 3S, 4R, 5R, 8R, 10R, 11R, 12S, 13S, 14R)-2-ethyl-3,4,10 -Trihydroxy-3,5,8,10,12,14-hexamethyl-11-[[3,4,6-trideoxy-3-(dimethylamino)-2-O-[(phenyl Methoxy)carbonyl]-β-hexylopyranosyl]oxy]-1-oxa-6-azacyclopentadecane-13,15dione.
[0282] method 1
[0283] Dimethyl sulfoxide / trifluoroacetic anhydride oxidation
[0284] Add 10g (0.014moL) of compound 3 into 60mL of dichloromethane under nitrogen protection, stir, cool to about 0°C, add 22.0mL of dimethyl sulfoxide (Chengdu Kelong Chemical Company, batch number: 20100708), and then take 4.6mL Trifluoroacetic anhydride (Jiangsu Shiyan Chemical Company, batch number: 20101008) was added dropwise, and after the dropwise addition was completed, it was reacted for 30 min, and 9.8 mL of triethylamine (Chengdu Kelong Chemical Company, batch number: 20100708) was added dropwise, and the inner temperature was controlled at -...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com