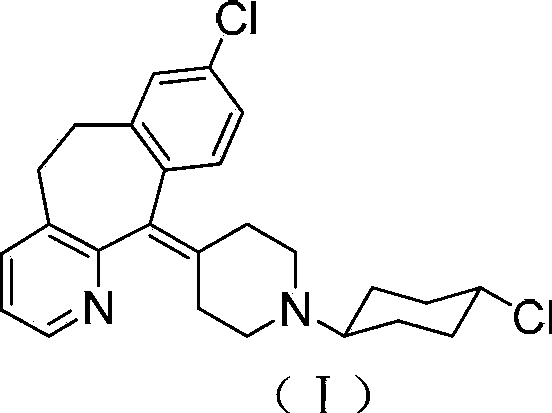
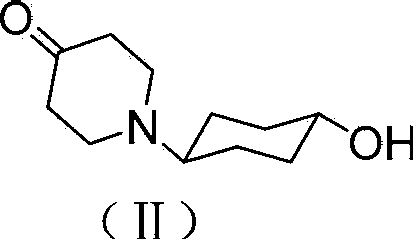
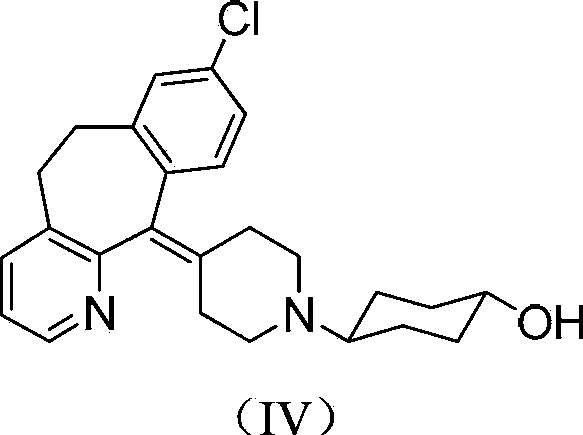
Tricyclic compound with antihistamine activity, preparation method and application
A compound, antihistamine technology, applied in the fields of organic chemistry, drug combination, allergic diseases, etc., can solve problems such as cardiovascular system impact, and achieve the effect of low toxic side effects and strong antihistamine activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Add 20mL of methanol and methyl acrylate (2.58g, 0.03mol) into a dry 50mL four-neck flask, and slowly add (S)-4-aminocyclohexanol (1.15g, 0.01mol) dropwise under stirring at room temperature, After dripping, the oil bath was heated to 40°C, reacted for 14 hours, and the excess methyl acrylate was evaporated under reduced pressure to obtain a light yellow oil. The crude product was separated and purified by silica gel column chromatography to obtain 2.62 g of the addition product, and the molar yield was 91.4%. .
[0035] Its structural formula is
[0036]
[0037] 1 H NMR (CDCl 3 ,500MHz)δ:3.64(s,6H),3.52(d,J=4.0Hz,1H),2.73(t,J=6.0Hz,4H),2.39(t,J=6.0Hz,5H),1.98( d,J=10.5Hz,2H),1.69(d,J=12.5Hz,2H),1.31~1.23(m,4H); 13 CNMR (CDCl 3 ,125MHz) δ:173.34,70.66,59.24,51.68,46.50,35.00,34.76,26.65.
[0038] Experiments have proved that the addition product can also be obtained by substituting ethanol, dichloromethane or ethyl acetate for methanol in this step.
[00...
Embodiment 2
[0064] Example 2: Antiasthmatic effect of compound (I) on guinea pigs with histamine-induced asthma
[0065] Take guinea pigs, place them in a plexiglass bell jar, spray them with saline histamine solution (0.8mg / mL) for 40s by ultrasonic atomization, record the time when guinea pigs develop asthma, take the time of convulsions and falls as the incubation period, and guinea pigs that exceed 180s will not be treated. Selected, 30 selected guinea pigs were randomly grouped, 10 in each group, divided into 3 groups: normal control group, desloratadine group (1mg / kg), compound (I) group (0.01mg / kg). Oral administration with 2mL / kg bw, 60 minutes after administration, put them into glass bell jars respectively, spray histamine hydrochloride solution according to the same conditions as pre-selection, record the incubation period of asthma, if no asthma occurs after 6 minutes, In terms of 6 minutes, the results were statistically processed.
[0066] The results are shown in Table 1 ...
Embodiment 3
[0070] Example 3: Effects on Muscle Tension of Guinea Pig Isolated Ileum Smooth Muscle
[0071] Guinea pigs (48 guinea pigs) were stunned with a wooden stick, and the abdomen was immediately opened, and the ileum about 15 cm long was separated, and the contents of the intestinal segment were washed with Tyrode's solution, and placed in Tyrode's solution at a constant temperature of 37°C for later use. oxygen. Cut the experimental intestinal tube (length 1cm) into 20mL Tyrode's solution at a constant temperature of 37°C, continue oxygenation, fix one end on the ventilation hook, and connect the other end to the muscle tension transducer and lead it to the computer interface, use BL The muscle tension value of the ileal smooth muscle was recorded systematically, and the experiment was carried out after the contraction of the intestinal segment was stable.
[0072] After the ileal contraction curve stabilized, record the tension value before adding the drug, then add the drug or...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com