Cell culture vessel and cell culture device
A cell culture and container technology, applied in the field of cell culture containers and cell culture devices, can solve the problems of multi-labor and cost, biological pollution, difficult double-layer culture, etc., and achieve the effect of safe recovery and inhibition of invasion.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051]
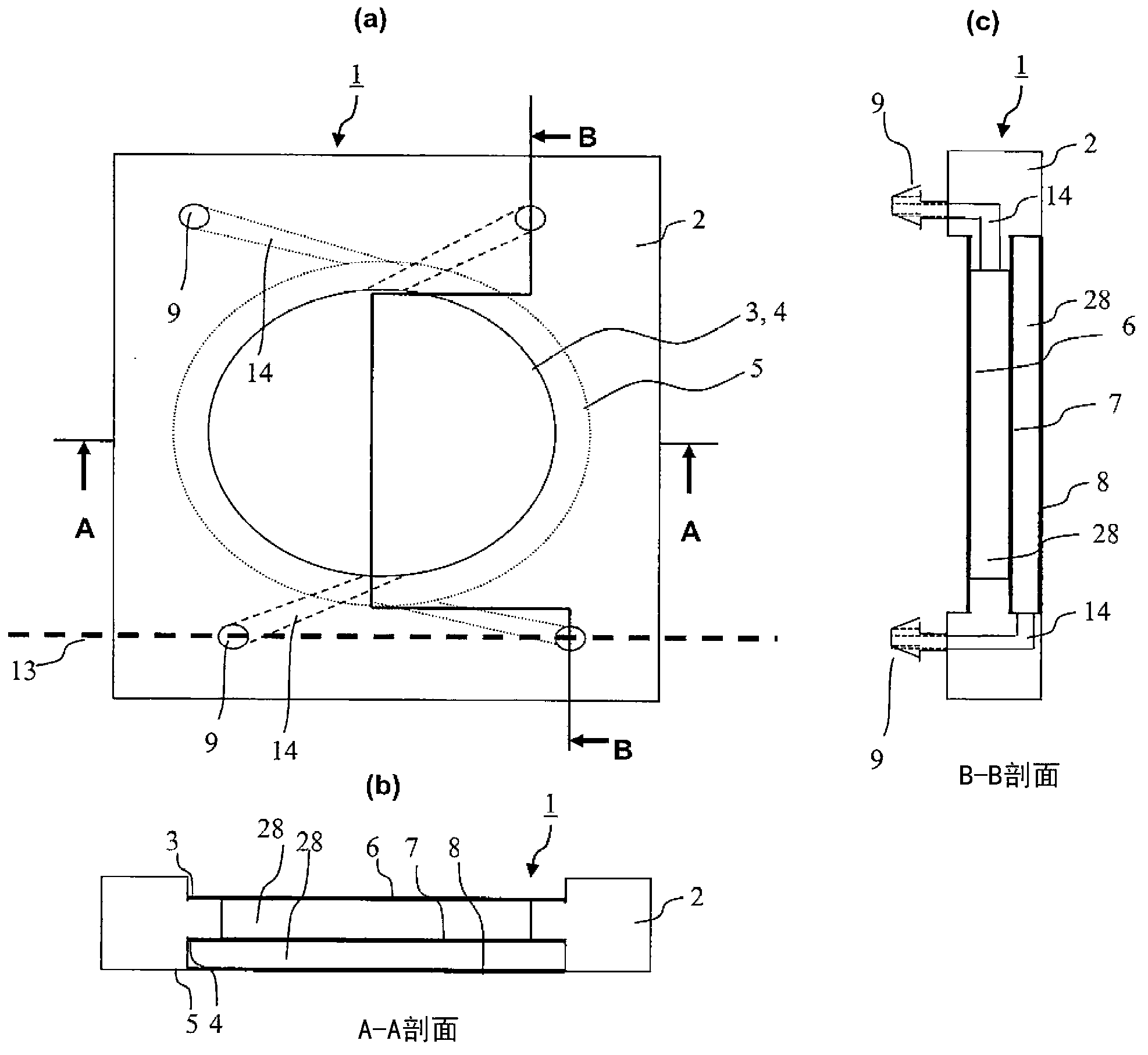
[0052] figure 1 It is a figure which shows an example of the structure of the cell culture container in 1st Example.
[0053] figure 1 (a) is a plan view, (b) is its A-A sectional view, and (c) is its B-B sectional view. The cell culture container 1 is a square and flat container made of plastics such as polycarbonate, polystyrene, polypropylene, etc. having both plasticity and rigidity. The frame body 2 is formed by injection molding or the like, and round portions 3 , 4 and 5 are formed therein. Furthermore, a gas permeable membrane 6 is welded to the circular portion 3 , a substance permeable membrane 7 is welded to the circular portion 4 , and a gas permeable membrane 8 is welded to the circular portion 5 . For welding the gas permeable membranes 6, 8 and the substance permeable membrane 7 to the circular parts 3, 5, 4, thermal welding or ultrasonic welding without using an adhesive that affects cell survival is preferable. By the above-mentioned layers and ...
specific example 1)
[0077]
[0078] First, made by injection molding figure 1 Frame 2 shown (material polycarbonate). Figure 12 The appearance of the frame body 2 actually produced is shown. By ultrasonic welding, the figure 1 The gas permeable film is welded on the round parts 3 and 5 of the figure 1 The material permeable membrane is welded on the circular part 4 (ultrasonic welding machine uses 2000Xdt40 manufactured by Branson Company: 0.8). deposited on figure 1The membranes of the circles 4 and 5 were hydrophilized by a plasma device (PC-300 manufactured by Samco was used for the plasma device). Figure 13 It shows the appearance of the state where the culture solution is kept in the container 1 after each film is welded.
[0079]
[0080] Next, a method for culturing corneal epithelial cells using the prepared cell culture container will be described. On the day before culturing corneal epithelial cells, as feeder cells, NIH-3T3 cells were treated with Mitomycin C (10 μg / ml) at ...
specific example 2)
[0097]
[0098] A temperature-responsive culture surface is produced by electron beam polymerization of N-isopropylacrylamide, which is a temperature-responsive high molecular weight monomer, on the cell culture surface of a closed system culture container. After confirming that the adhesion and detachment of corneal epithelial cells were normally carried out on the culture surface, corneal epithelial tissues were produced in the same manner as in Example 1.
[0099]
[0100] On the 16th day of culture, replace with fresh culture medium at room temperature, and let stand at room temperature (about 25°C) for 30 minutes. Then, using a ring-cut hydrophilic PVDF membrane (manufactured by Millipore) as a supporting membrane, the cell sheet was peeled off from the culture surface and recovered.
[0101]
[0102] It carried out similarly to specific example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com