Preparation method of FGF1 (fibroblast growth factor 1) full-length protein by utilizing escherichia coli
A technology of Escherichia coli and full length, applied in the field of preparing FGF1 full length protein from Escherichia coli, can solve the problems of low solubility and low product purity, and achieve the effect of high solubility and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
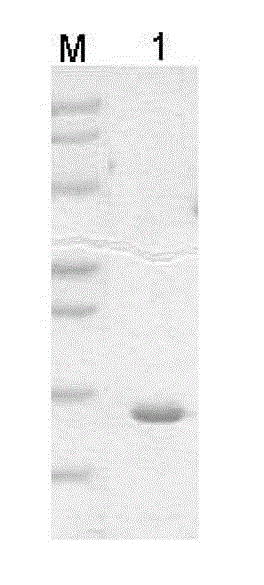
[0046] Transform the recombinant plasmid with correct sequencing into Escherichia coli BL21 according to the above steps, cultivate 2L of the bacteria at 37°C, induce the expression of the bacteria after 4 hours under the condition of OD500 of 0.6 and IPTG of 1mM, centrifuge and resuspend the bacterial pellet in Centrifuge at 5500rpm at 4°C for 15 min in 50 mL of 4°C pre-cooled cell washing buffer, then resuspend the pellet in 25 mL of 4°C pre-cooled cell lysate, add PMSF to a final concentration of 1 mmol / L, stir at room temperature for 15 min, and After centrifuging at 12000rpm for 10min at 4°C, collect the bacterial pellet; resuspend the collected bacterial pellet again, add 0.1mmol / L MgCl pre-cooled at 4°C 2 The solution was 200mL, stirred at 4°C for 10min, centrifuged at 12000rpm for 10min, and then added 10mmol / L Tris-Cl (pH7.1) to the supernatant obtained by centrifugation until the pH reached 7.1. -Cl (pH7.1), dialysis at 4°C to obtain the full-length protein of FGF1 c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com