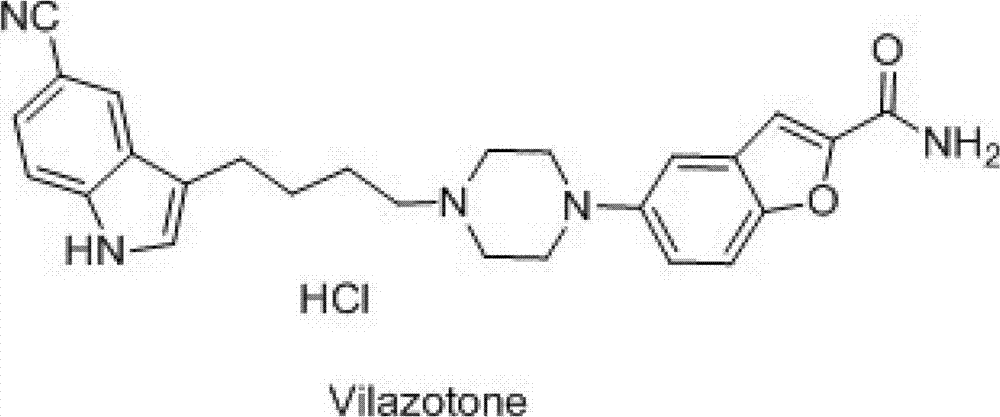
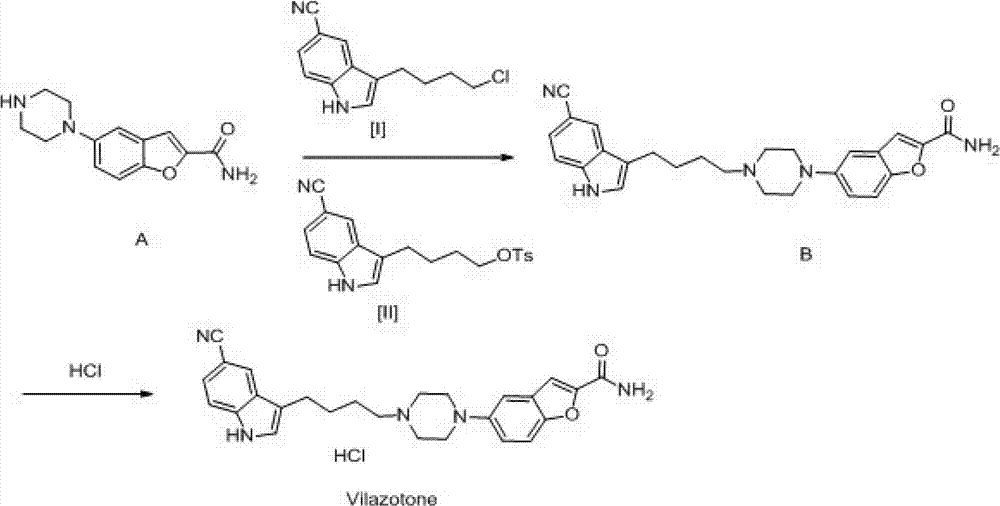
Synthesis method of 5-(piperazino-1-yl)benzofuryl-2-formamide
A technology of benzofuran and synthetic method, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of low product yield and purity, long synthetic route, etc., and achieve high product yield and purity, simple and convenient production process, and good product quality Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] 1) Preparation of 4-(piperazin-1-yl) salicylaldehyde (II)
[0050] At room temperature, add 1000 mL of anhydrous acetonitrile to the reaction flask under the protection of nitrogen, then add 37.25 g of 4-piperazin-1-yl-phenol, MgCl 2 30.1g, 110mL of triethylamine, 31.35g of paraformaldehyde, heat up and reflux for 5 hours, wait for the reaction solution to cool to room temperature, slowly pour the reaction solution into 1L of hydrochloric acid with a concentration of 5%, and mix the liquid with 1L of dichloro Methane was extracted twice, the water phase was adjusted to PH to 8 with 10% NaOH aqueous solution, a large amount of solids were precipitated, filtered with suction, the filter cake was washed twice with a small amount of water to remove inorganic salts, and the filter cake was air-dried at 55~65°C. 37.5 g of a light yellow solid was obtained with a yield of 88% and a purity of 98%.
[0051] 2) Preparation of ethyl 5-(piperazin-1-yl)benzofuran-2-carboxylate (II...
Embodiment 2
[0056] 1) Preparation of 4-(piperazin-1-yl) salicylaldehyde (II)
[0057] At room temperature, add 900 mL of tetrahydrofuran to the reaction flask under the protection of nitrogen, then add 44.2 g of 4-piperazin-1-yl-phenol, MgCl 2 35.6g, 135mL pyridine, 37.2g paraformaldehyde, heat up and reflux for 4 hours, wait for the reaction solution to cool to room temperature, slowly pour the reaction solution into 1L hydrochloric acid with a concentration of 5%, and extract the mixed liquid with 1L dichloromethane Twice, the water phase was adjusted to PH to 8 with 10% NaOH aqueous solution, a large amount of solids were precipitated, filtered with suction, the filter cake was washed twice with a small amount of water to remove inorganic salts, and the filter cake was air-dried at 55~65°C to obtain 37.8 g of pale yellow solid, yield 86.5%, purity 98.5%.
[0058] 2) Preparation of ethyl 5-(piperazin-1-yl)benzofuran-2-carboxylate (III)
[0059] At room temperature, add 425 mL of DMF ...
Embodiment 3
[0063] 1) Preparation of 4-(piperazin-1-yl) salicylaldehyde (II)
[0064] At room temperature, add 1000mL of toluene to the reaction flask under the protection of nitrogen, then add 42.5g of 4-piperazin-1-yl-phenol, MgCl 2 33.6g, 165mL of diisopropylethylamine, 35.8g of paraformaldehyde, heat up and reflux for 7 hours, wait for the reaction solution to cool to room temperature, slowly pour the reaction solution into 1L hydrochloric acid with a concentration of 5%, and use the mixed liquid with Extract twice with 1L dichloromethane, adjust the pH of the water phase to 8 with 10% NaOH aqueous solution, precipitate a large amount of solids, filter with suction, wash the filter cake twice with a small amount of water to remove inorganic salts, blow the filter cake at 55~65°C After drying, 37.2 g of a light yellow solid was obtained, with a yield of 75.6% and a purity of 98%.
[0065] 2) Preparation of ethyl 5-(piperazin-1-yl)benzofuran-2-carboxylate (III)
[0066] At room temper...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com