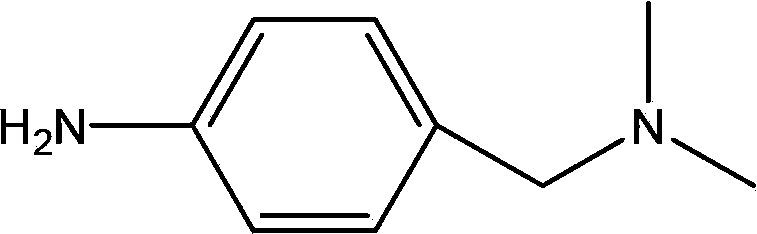
Process for preparing 4-amino-N, N-dimethylbenzylamine
A technology of dimethylbenzylamine and dimethylamine, which is applied in the field of preparation of 4-amino-N,N-dimethylbenzylamine, can solve the problems of low yield, large pollution, long reaction time, etc. Ease of implementation, optimized reaction conditions, high purity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
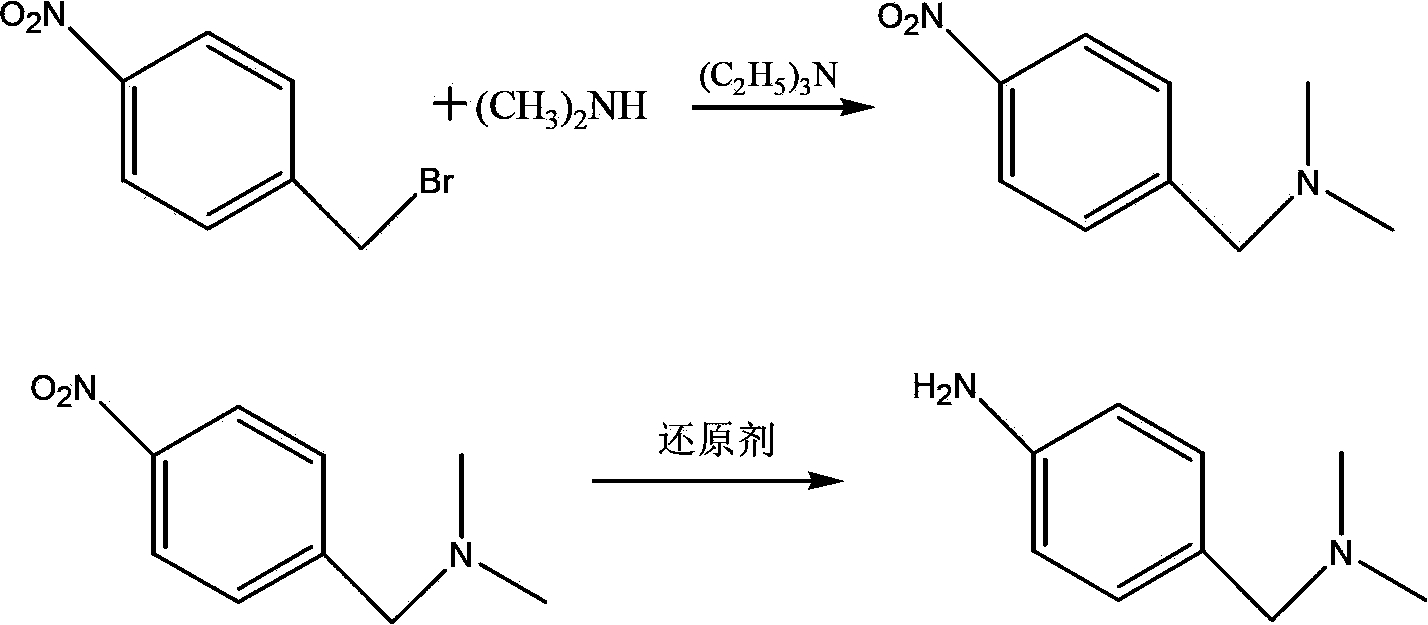
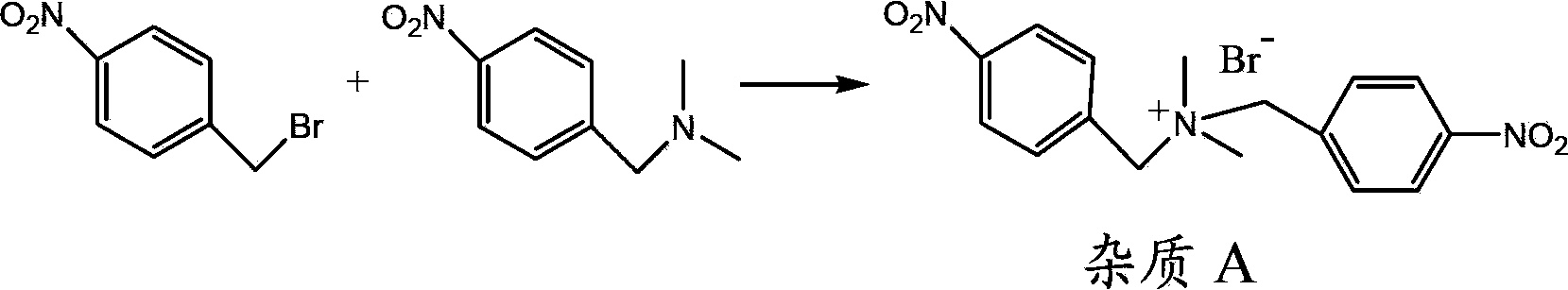
[0052] In a 500mL four-necked flask, add 150mL of chloroform without water, 32g (0.379 moles) of dimethylamine hydrochloride, stir, cool to 5°C, add 59g (0.584 moles) of triethylamine dropwise, and then every 10 minutes Add 5.7g, 5.3g, 4.9g, 4.5g, 4.1g, 3.8g, 3.5g, 3.2g, 1.0g of p-nitrobenzyl bromide in batches to ensure the single batch feeding amount of p-nitrobenzyl bromide in the reaction system The mole percentage of dimethylamine in the reaction system is less than 7%, and 36g (0.167 mole) of p-nitrobenzyl bromide is added. The temperature was controlled at 15°C, and the reaction was completed after stirring for 2 hours after the addition. The GC content of 4-nitro-N,N-dimethylbenzylamine is 98.3%, and the impurity A is 1.5%. Add 150 mL of water, stir and separate layers, remove the water phase, wash the organic phase with water, concentrate to dry the solvent, add 100 mL of n-heptane to the residue, cool at -20°C for 6 hours, filter, remove the filter cake, and distill...
Embodiment 2
[0057] In a 500mL four-necked flask, add 150mL of chloroform without water, 32g (0.379 moles) of dimethylamine hydrochloride, stir, cool to 5°C, add 59g (0.584 moles) of triethylamine dropwise, and then every 10 minutes Add p-nitrobenzyl bromide 3.2g, 3.0g, 2.8g, 2.6g, 2.4g, 2.3g, 2.2g, 2.1g, 2.0g, 1.9g, 1.8g, 1.7g, 1.6g, 1.5g, 1.4g, 1.3g, 1.2g, 1.0g, to ensure that the single batch of p-nitrobenzyl bromide in the reaction system and the molar percentage of dimethylamine in the reaction system are all less than 4%, add 36g of p-nitrobenzyl bromide altogether (0.167 mol). The temperature was controlled at 15°C, and the reaction was completed after stirring for 2 hours after the addition. The GC content of 4-nitro-N,N-dimethylbenzylamine is 99.3%, and the impurity A is 0.5%. Add 150 mL of water, stir and separate layers, remove the water phase, wash the organic phase with water, concentrate to dry the solvent, add 100 mL of n-heptane to the residue, cool at -20°C for 6 hours, ...
Embodiment 3
[0062] In a 500mL four-neck flask, add 150mL of chloroform without water, 34g (0.402 moles) of dimethylamine hydrochloride, stir, cool to 5°C, add dropwise 50g (0.494 moles) of triethylamine, and then drop in 3 hours Add a solution of 36 g (0.167 moles) of p-nitrobenzyl bromide dissolved in 100 mL of chloroform, control the temperature of the reaction system at 15° C., and stir for 2 hours after the addition. After the reaction is complete, add 150 mL of water, stir and separate the layers, remove the water phase, wash the organic phase with water, concentrate to dry the solvent, add 100 mL of n-heptane to the residue, cool at -20°C for 6 hours, filter, remove the filter cake, and distill the filtrate to remove the solvent. 28.8 g of 4-nitro-N,N-dimethylbenzylamine was obtained, the yield was 96%, the GC content was 99.8%, and impurity A was not detected.
[0063] 1 H-NMR data: 1 H-NMR (400MHz, CDCl 3 )δ:2.26(6H,s),3.51(2H,s),7.49(2H,d,J=9.0Hz),8.18(2H,d,J=8.7Hz)
[0064] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com