Preparation method for gold-silver alloy nanometer particles
A gold-silver alloy and nanoparticle technology, applied in the field of material chemistry, can solve problems such as surface susceptibility, affecting SERS effect, etc., and achieve the effects of short time consumption, increased active sites, and strong Raman enhancement effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
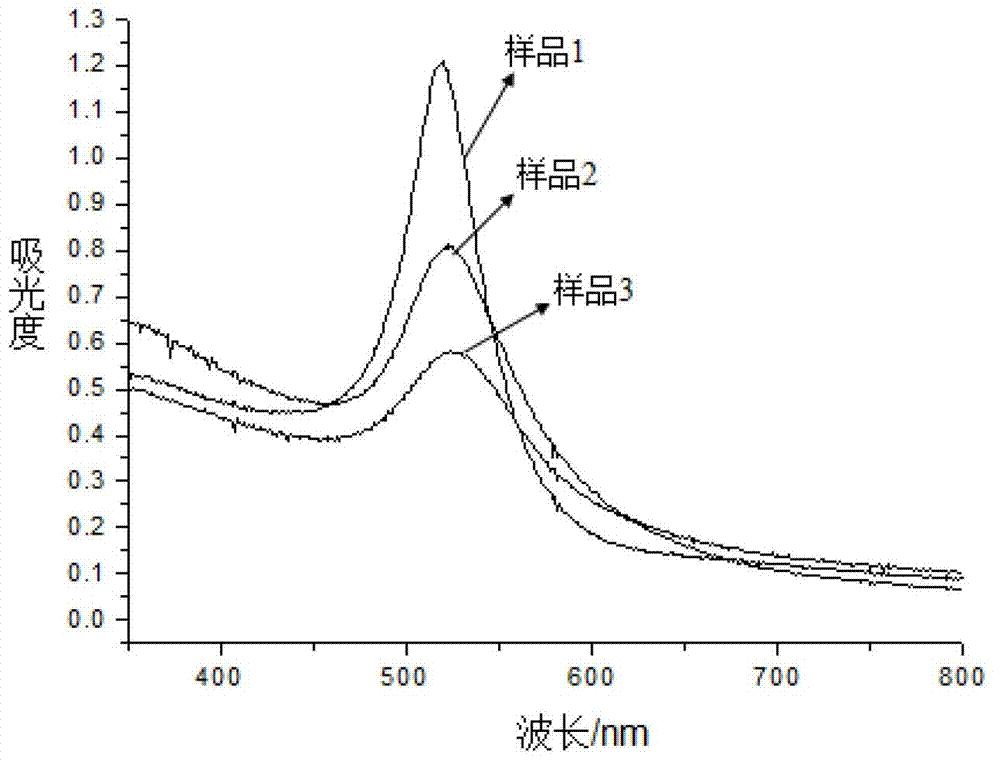
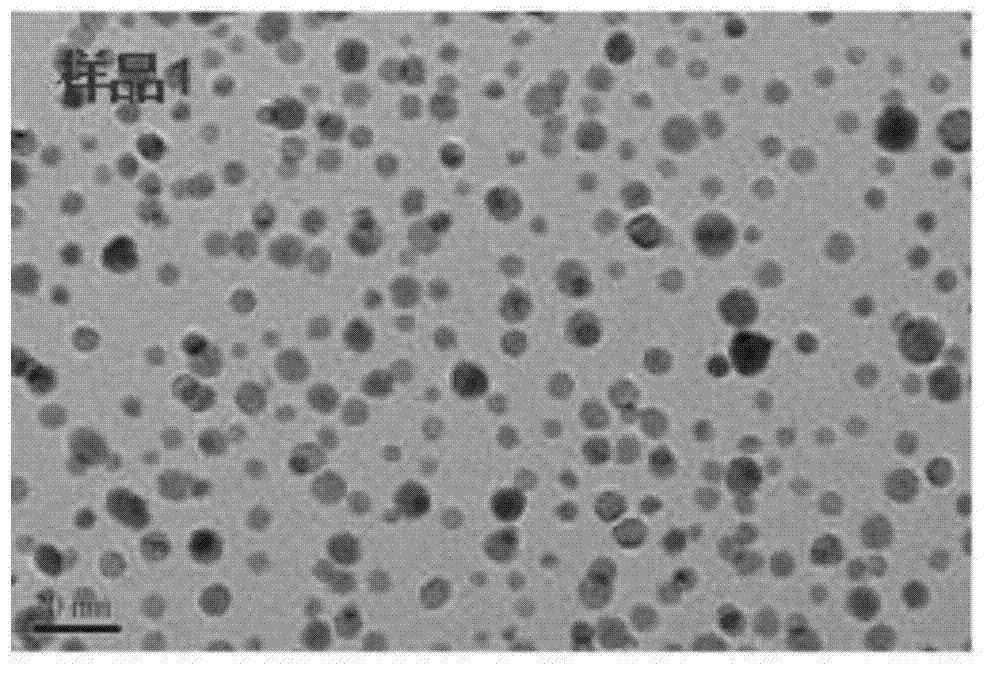
[0026] Mix 7.5mL of chloroauric acid with a concentration of 0.2428mmol / L and 22.5mL of silver nitrate, put it into a beaker, stir continuously and heat to 90°C, add 4mL of sodium phytate under slight boiling, the concentration of sodium phytate is 10 -3 mol / L, continue stirring and heating for 10 minutes, add 0.2 mL of trisodium citrate with a mass concentration of 1%, and heat for 1 hour under constant stirring at a heating temperature of 90°C, the color of the solution turns light red. This reaction solution was labeled as sample 1 and stored at 4°C.
Embodiment 2
[0028] The chloroauric acid solution and silver nitrate in embodiment 1 are mixed according to the ratio of 1:1 according to the volume ratio, namely chloroauric acid solution 15mL, silver nitrate solution is also 15mL, other preparation conditions are all constant, make red solution, will The reaction solution was marked as sample 2 and stored at 4°C.
Embodiment 3
[0030] The chloroauric acid solution and silver nitrate in Example 1 are mixed according to the volume ratio of 3:1, i.e. chloroauric acid solution 22.5mL, silver nitrate solution is also 7.5mL, other preparation conditions are all constant, dark red color is obtained solution, the reaction solution was labeled as sample 3, and stored at 4°C.
[0031] Characterization of gold-silver alloy nanoparticles:
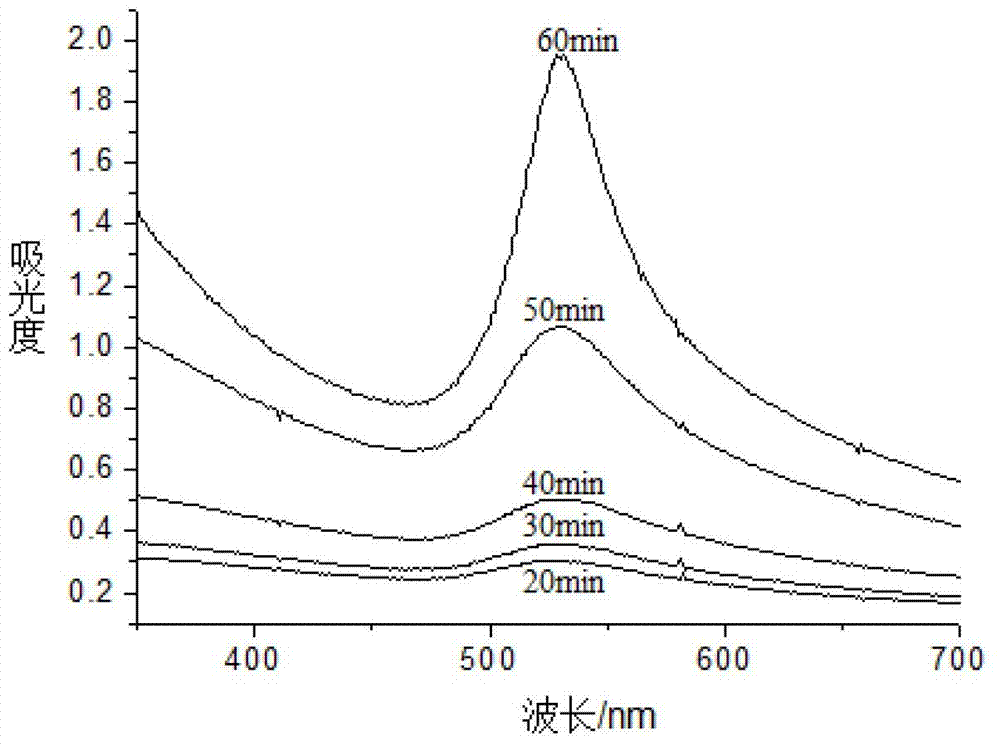
[0032] (1) Changes in UV-Vis absorption spectrum during the preparation of alloy nanoparticles of sample 3
[0033] During the preparation of sample 3, after adding 0.2 mL of trisodium citrate to the mixed solution, a reduction reaction occurred in the solution for a total of 1 hour. Sampling and analysis were carried out in five different reaction time periods when the reduction reaction occurred in 20 minutes, 30 minutes, 40 minutes, 50 minutes and 60 minutes, and the samples were put into the ultraviolet spectrophotometer for detection. Specific as figure 1 shown. As c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com