Medical application of przewaquinone A
A technology of purple salvia and A, which is used in pharmaceutical formulations, medical preparations containing active ingredients, antitumor drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
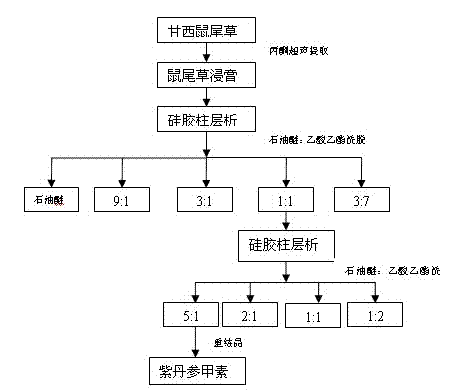
[0019] Embodiment 1: Preparation of purple danshenin (the purple danshenin used in the following examples is prepared by the following method)
[0020] In the experiment, 7.3 kg of dried sage (whole herb) was used, crushed through a 60-mesh sieve, ultrasonically extracted 5 times with 100% acetone (20 L each time) at room temperature, and the extract was concentrated under reduced pressure to obtain 300 g of crude extract; Put the obtained crude extract on a normal-phase silica gel column (80-100 mesh, Qingdao Ocean Chemical) (dry loading), and sequentially use pure petroleum ether, petroleum ether-ethyl acetate mixture (petroleum ether: ethyl acetate (9:1, 3:1, 1:1, 3:7) were eluted, and each part of the eluate was collected, concentrated under reduced pressure, and the concentrated solution was separately transferred to a 10mL penicillin bottle, and detected by TLC, the components were roughly the same The cuts of petroleum ether: ethyl acetate=1:1 eluent elution part (52 g)...
Embodiment 2
[0024] Example 2: Effect of Danshen A on the proliferation of human liver cancer cell line HepG-2 (MTT)
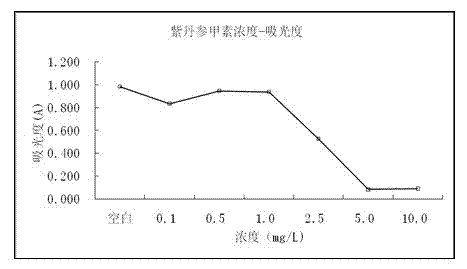
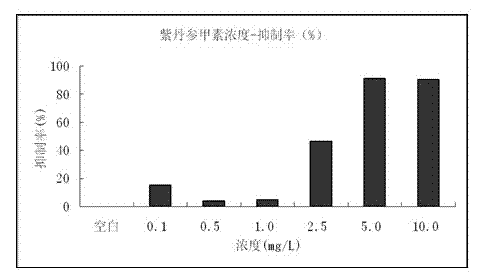
[0025] The human liver cancer cell line HepG-2 was incubated at 37°C in 5% CO 2 After culturing under the above conditions for 24 hours, inoculate them into a 96-well plate at a density of 3000 cells per well. , 10.0 mg / L of different concentrations of liquid medicine, and added to a 96-well plate, at 37 ℃, 5% CO 2 The culture was continued for 72 hours under the condition of 2, 20 μL of MTT (3-(4,5-dimethylthiazole-2)-2,5-diphenyltetrazolium bromide) was added to each well and the incubation was continued for 4 h, and the upper To the supernatant, 150 μL DMSO was added to each well, shaken for 1 h, and the absorbance OD value was measured at 490 nm on a microplate reader. (See figure 2 )
[0026] Experimental results such as image 3 It was shown that when the concentration of salvianolin A was greater than 2.5 mg / L, the proliferation activity of cells decreased si...
Embodiment 3
[0027] Example 3: Experiment of the inhibitory effect of purple danshen A on angiogenesis of chicken embryo allantoic membrane
[0028] Take fresh eggs at a temperature of 37.8 °C, CO 2The concentration was 5%, and the humidity was suitable for incubation for 7 days. A window (1×1 cm) was opened at the end of the air chamber, and a sterile filter paper sheet with a diameter of 6 mm was used as the drug delivery carrier, which was placed on the allantoic membrane. Administration groups with different concentrations of 5.0, 10.0, and 15.0 mg / L were administered, and a blank group (normal saline group) and a positive control drug group (dexamethasone group) were set up, and the window was sealed with scotch tape, and incubation was continued under the above conditions. After 48 hours, the allantoic membrane tissue was removed, put into dehydration solution (methanol:acetone=1:1) for dehydration and fixed for 15 min, and the blood vessels on the surface of the allantoic membrane t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com