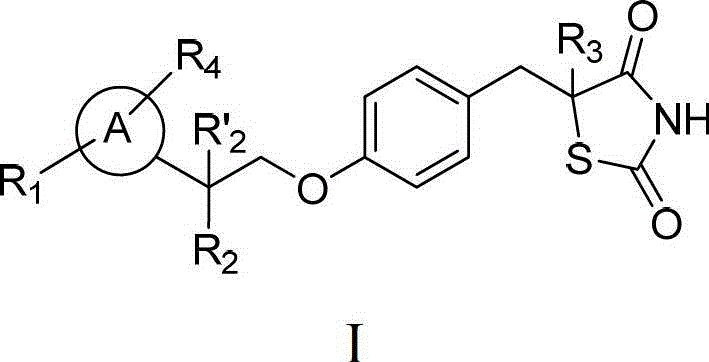
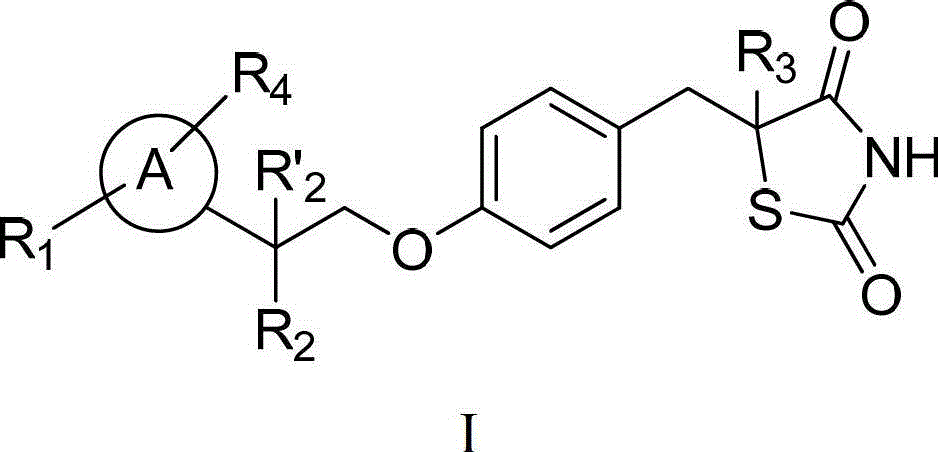
Thiazolidinedione analogues
A technology of drugs and compositions, applied in the field of thiazolidinedione analogues, capable of solving problems such as unsatisfactory side effects and sodium reabsorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0174] Example 1: 5-[4-(2-oxo-2-phenylethoxy)benzyl]-1,3-thiazolidine-2,4-dione.
[0175]
[0176] step 1: Preparation of 4-(2-hydroxy-2-phenylethoxy)benzaldehyde.
[0177] Add toluene (85ml), 4-hydroxybenzaldehyde (9.89g, 81.0mmol), PEG4000 (polyethylene glycol, 1.15g ) and 1M NaOH (85ml) and the stirred mixture was heated at 78°C overnight. After cooling to room temperature, the reaction mixture was extracted with EtOAc, and the organic phase was washed with brine, dried (Na 2 SO 4 ), filtered and evaporated in vacuo. The resulting yellow oil was chromatographed on a medium silica gel column eluting with 0-10% EtOAc / DCM. will mainly contain higher R f Fractions from the spot were combined and evaporated in vacuo to give 1.85 g of the title compound as a yellow oil. will mainly contain lower R f Fractions from the spot were combined and evaporated in vacuo to give 0.64 g of the regioisomer as a colorless viscous oil. The combined fractions were combined and rechr...
Embodiment 2
[0184] Example 2: Preparation of 5-{4-[2-(4-fluorophenyl)-2-oxoethoxy]benzyl}-1,3-thiazolidine-2,4-dione.
[0185] step 1: Preparation of 4-[2-(fluorophenyl)-2-hydroxyethoxy]benzaldehyde
[0186] To a stirred solution of 2-(4-fluorophenyl)oxirane (5.60 g, 40.0 mmol) in toluene (65 ml) was added 4-hydroxybenzaldehyde (7.40 g, 61.0 mmol), 1M NaOH (65 ml ) and PEG4000 (polyethylene glycol, 0.85 g) and the reaction mixture was heated at 78° C. overnight. After cooling to room temperature, the reaction mixture was extracted with EtOAc (2x150ml) and the combined extracts were washed with brine, dried (Na 2 SO 4 ), filtered and evaporated in vacuo. The resulting light brown oil was chromatographed on silica gel (eluting with 30-40% EtOAc / hexanes). will contain higher R f The spot fractions were combined and evaporated in vacuo to give 2.38 g of the regioisomer of the product as a white solid. will contain lower R f The spot fractions were combined and evaporated in vacuo to ...
Embodiment 3
[0193] Example 3: Preparation of 5-{4-[2-(2-fluorophenyl)-2-oxoethoxy]benzyl}-1,3-thiazolidine-2,4-dione.
[0194]
[0195] step 1: Preparation of 2-(2-fluorophenyl)oxirane.
[0196] Add o-fluorostyrene (5.0g, 41.0mmol) and acetic acid (2.33mL, 40.9mmol) in dioxane (33ml) and H at 0°C 2 To the solution in O (78ml) was added N-bromosuccinimide (8.02g, 45.0mol) in three portions. The reaction mixture was warmed to room temperature and stirred overnight. Sodium carbonate (8.68 g, 81.9 mmol) was added portionwise, followed by 1M NaOH (ca. 10 ml) and the reaction mixture was stirred at room temperature overnight. The reaction mixture was partitioned between water and EtOAc, and the aqueous phase was extracted with EtOAc. The combined organic phases were washed with brine, dried (Na 2 SO 4 ), filtered and evaporated in vacuo to give 5.31 g of the title compound as a slightly colored oil which was used without further purification. C 8 h 7 MS(ESI+)m / z138.1(M+H) of FO + ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com