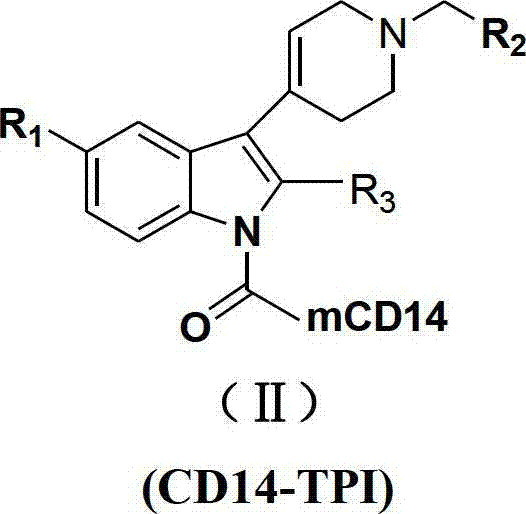
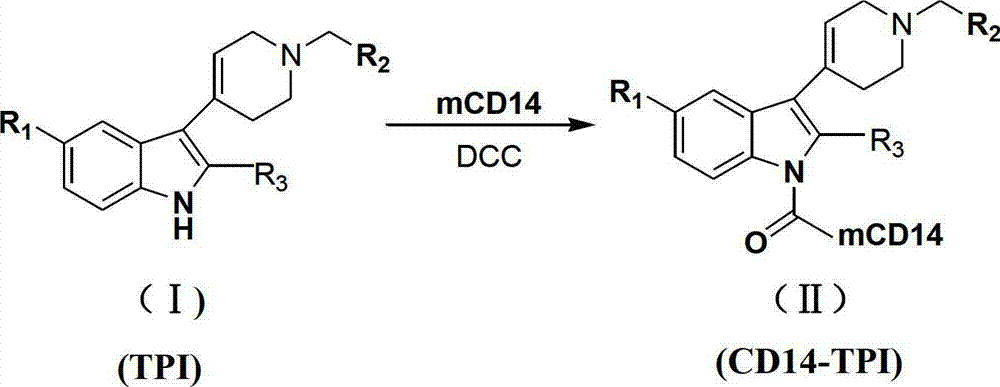
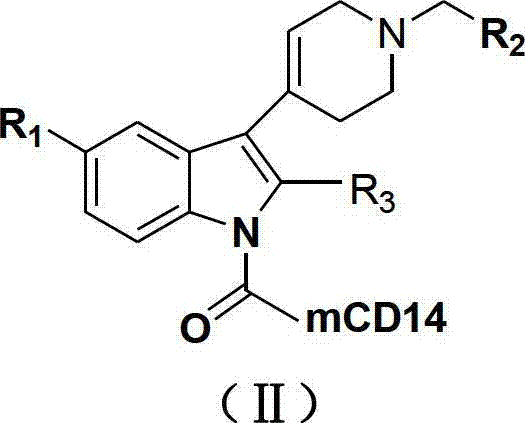
N-substituted-tetrahydropyridylindole-monoclonal antibody CD14 conjugates, and preparation method and application thereof
A technology of tetrahydropyridine indole and tetrahydropyridine indole, applied in the field of chemical and biological medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: the preparation method of conjugate (IIa)~(IIr)
[0049] For the preparation methods of compounds (Ia)~(Ij), please refer to related patents: patent application number: 201110224878.x, publication number: CN102276581A.
[0050] For the preparation methods of compounds (Ik)~(Ir), see Example 2~Example 9 of this patent specification.
[0051] Take 10 mg of compounds (Ia)~(Ir), dissolve them in 0.1 mL DMSO respectively, add 0.1 mL of DCC solution with a concentration of 0.1 mol / L, and 0.1 mL of CD14 with a concentration of 2 μg / mL to the above solutions, and then use Physiological saline was used to dilute the above mixed solution to 1 mL. After reacting at 37°C for 30 minutes, the conjugates (Ⅱa)~(Ⅱr) were obtained.
[0052] The killing effects of the conjugates (Ⅱa)~(Ⅱr) on leukemia K562 cells were measured by MTT method, and multiple groups of control groups were established at the same time. The control group included: blank group, CD14+DMSO+DCC, CD14,...
Embodiment 2
[0053] Example 2: Preparation method of 3-[N-(2-tetrahydrofurylmethyl)-1,2,3,6-tetrahydropyridin-4-yl]indole (Ik):
[0054] Add 0.005mol indole, 5mL methanol and 5mL sodium methoxide solution (30wt%CH 3 OH solution), under the condition of ice-water bath, stir and add the mixed solution of 0.01mol N-(2-tetrahydrofurylmethyl)-4-piperidone and 5mL methanol. After the dropwise addition, the mixture was stirred at room temperature for 20 min, and then heated to reflux at 63° C. for 5 h, a yellow solid was precipitated, and the reaction progress was tracked by thin-layer chromatography (TLC). After the reaction, the solid was washed with absolute ethanol and recrystallized with ethyl acetate to obtain 3-[N-(2-tetrahydrofuranmethyl)-1,2,3,6-tetrahydropyridin-4-yl]ind Indole (Ik).
[0055] Yield 54%; yellow solid; mp162-164℃; 1 H NMR (400MHz, DMSO-d 6 )δ11.08(s,1H),7.78(d,J=7.9Hz,1H),7.51-6.84(m,4H),6.08(d,J=3.4Hz,1H),4.13-3.86(m,1H ),3.85-3.50(m,2H),3.15(m,2H),2.68(m,2H);2.56-2...
Embodiment 3
[0056] Example 3: Preparation method of 3-[N-phenethyl-1,2,3,6-tetrahydropyridin-4-yl]indole (Il):
[0057] Add 0.005mol indole, 5mL methanol and 5mL sodium methoxide solution (30wt%CH 3 OH solution), and add a mixture of 0.01mol N-phenethyl-4-piperidone and 5mL methanol with stirring under ice-water bath conditions. After the dropwise addition, the mixture was stirred at room temperature for 20 min, and then heated to reflux at 63° C. for 6 h, a yellow solid was precipitated, and the reaction progress was tracked by thin layer chromatography (TLC). After the reaction, the solid was washed with absolute ethanol and recrystallized with ethyl acetate to obtain 3-(N-phenethyl-1,2,3,6-tetrahydropyridin-4-yl)indole (Il) .
[0058] Yield 64%; yellow solid; mp172-174℃; 1 H NMR (400MHz, DMSO-d 6 )δ11.18(s,1H),7.79(d,J=6.9Hz,1H),7.58-6.78(m,9H),6.13(d,J=2.4Hz,1H),3.53-3.46(m,4H ),3.25(m,2H),2.63(t,J=6.0Hz,2H);2.49(s,2H);IR(KBr):3072,2918,2876,1640,1613,1570,1485,1452,1436 ,1370,1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com