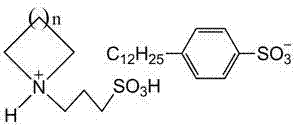
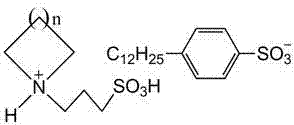
Ionic liquids with surface active function and preparation method for ionic liquids
A technology of ionic liquid and surface activity, which is applied in the preparation of sulfonate, chemical instruments and methods, organic chemistry, etc., can solve the problems of high energy consumption and achieve the effects of increasing the formation temperature, reducing the formation pressure, and shortening the induction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] When n=4, the nitrogen-containing heterocycle is cyclohexyl imine. Step 1: Dissolve 0.10 mol of 1,3-propane sultone in 50 ml of toluene, stir vigorously and add 0.10 mol of cycloheximide dropwise in an ice-water bath at 0°C, after the drop is complete, slowly heat up to 30°C for reaction After 8 h, the reactant was suction-filtered, the filter cake was washed three times with ethyl acetate, and dried at 85°C to obtain the white ionic liquid precursor 1-(3-sulfonic acid)propylcyclohexylimide ylide. Step 2: Dissolve 0.10 mol of 1-(3-sulfonic acid group) propylcyclohexyl imide ylide in water, stir at room temperature, add 0.10 mol of dodecylbenzenesulfonic acid dropwise, and set the temperature at 60°C after dropping React in a water bath for 30 h, filter the reactant to remove water under reduced pressure, and wash the obtained viscous liquid with ether and methanol to remove the precursor of the incomplete reaction. After washing, distill under reduced pressure to obtain...
Embodiment 2
[0028] When n=3, the nitrogen-containing heterocycle is piperidine. Step 1: Dissolve 0.15 mol of 1,3-propane sultone in 50 ml of toluene, stir vigorously and add 0.10 mol of piperidine dropwise in an ice-water bath at 0°C, after dropping, slowly raise the temperature to 70°C for 2 h , the reactant was suction-filtered, the filter cake was washed three times with ethyl acetate, and dried at 80°C to obtain the white ionic liquid precursor 1-(3-sulfonic acid)propylpiperidinium ylide. Step 2: Dissolve 0.11 mol of 1-(3-sulfonic acid) propylpiperidine ylide in water, stir at room temperature, add 0.11 mol of dodecylbenzenesulfonic acid aqueous solution dropwise, and place in a 95°C water bath after dropping Under the conditions of reaction for 2 h, after filtration, the reactant was distilled to remove water under reduced pressure, and the viscous liquid obtained was washed with ether and methanol respectively to remove the incomplete reaction precursor, and then distilled under red...
Embodiment 3
[0031] When n=2, the nitrogen-containing heterocycle is pyrrolidine. Step 1: Dissolve 0.10 mol of 1,3-propane sultone in toluene, stir vigorously and add 0.10 mol of pyrrolidine dropwise in a water bath at 35°C. After suction filtration, the filter cake was washed three times with ethyl acetate, and dried at 80°C to obtain the light yellow ionic liquid precursor 1-(3-sulfonic acid)propylpyrrolidinium salt. Step 2: Dissolve 0.10 mol of 1-(3-sulfonic acid) propylpyrrolidinium ylide in water, stir at room temperature, add 0.10 mol of dodecylbenzenesulfonic acid aqueous solution dropwise, and place in a 70°C water bath after dropping Under the conditions of reaction for 16 h, after filtration, the reactant was distilled to remove water under reduced pressure, and the obtained viscous liquid was washed with ether and methanol respectively to remove the incomplete reaction precursor, and then distilled under reduced pressure to obtain 1-(3-sulfonic acid Acid group) propylpyrrolidin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com