Pyrazolone derivative, amd application and preparation method of pyrazolone derivative
A preparation and compound technology, applied in the application field of quality control methods, can solve the problems of public reports without compound structure confirmation, quality decline of pharmaceutical preparations, and no method for preparing compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
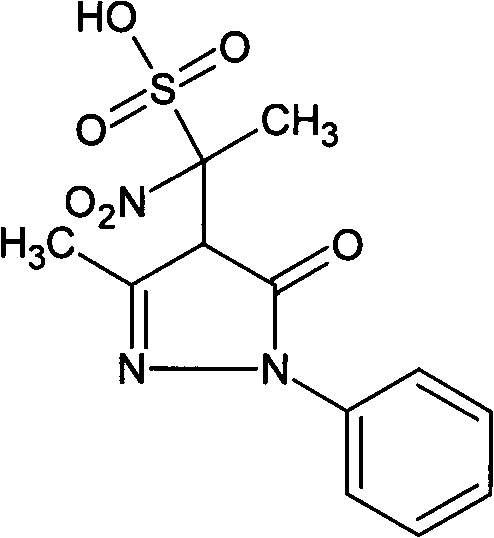
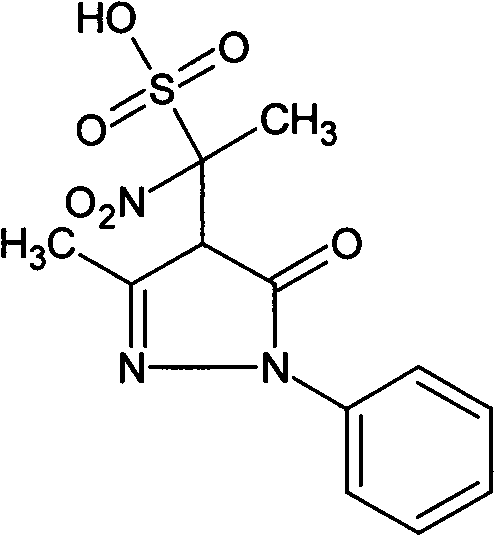
[0019] Embodiment 1: Preparation of the present invention compound 4-(1-nitro-1-sulfonic acid group) ethyl-3-methyl-1-phenyl-2-pyrazolin-5-one
[0020] Weigh 10 g of edaravone, 20 g of cysteine hydrochloride, and 10 g of sodium bisulfite into 4 L of water, and heat in a water bath at 98-100° C. for 24 hours. The reaction was completed, concentrated, filtered, and the filtrate was separated from the compound of the present invention by preparative liquid chromatography, the separated liquid containing the compound of the present invention was collected, and the separated liquid was concentrated to obtain 0.3 g of the compound of the present invention, with a yield of 3%.
[0021] The structure of the product is identified by nuclear magnetic resonance and single crystal X-ray diffraction. The results of nuclear magnetic resonance are as follows:
[0022]
[0023] NMR (DMSO-d 6 )
[0024]
[0025]
Embodiment 2
[0026] Embodiment 2: Determination of 4-(1-nitro-1-sulfonic acid group) ethyl-3-methyl-1-phenyl-2-pyrazolin-5 in Edaravone preparation by high performance liquid chromatography - ketone content
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com