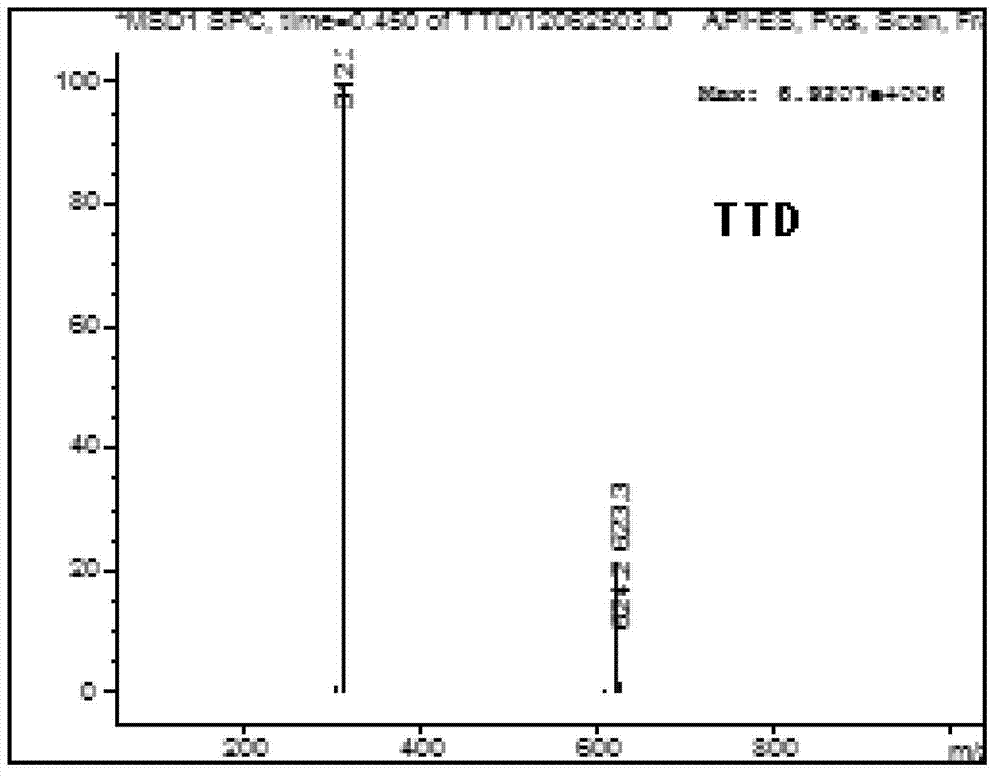
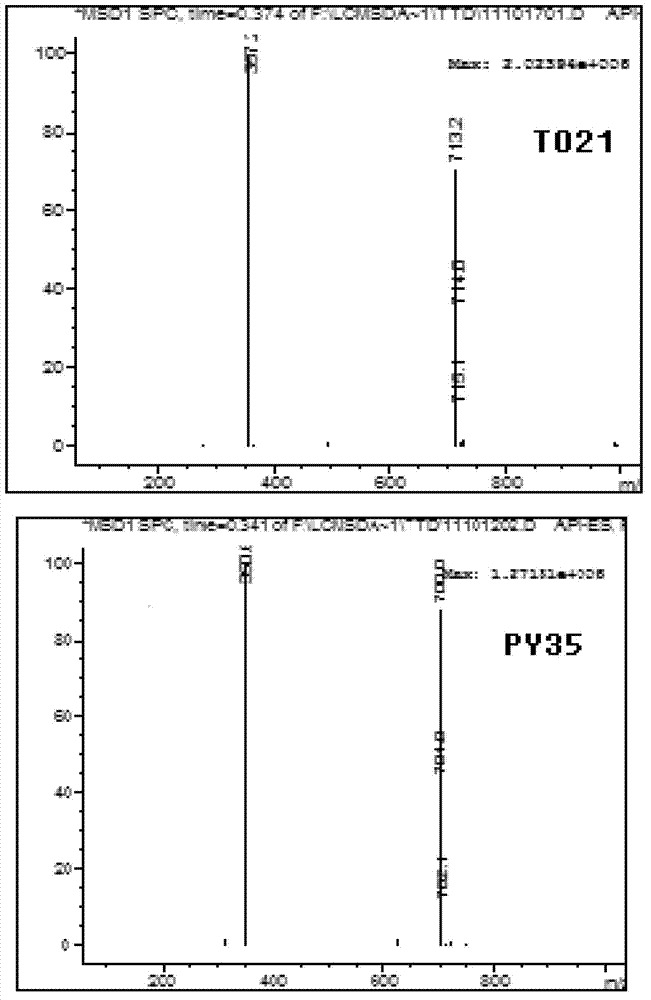
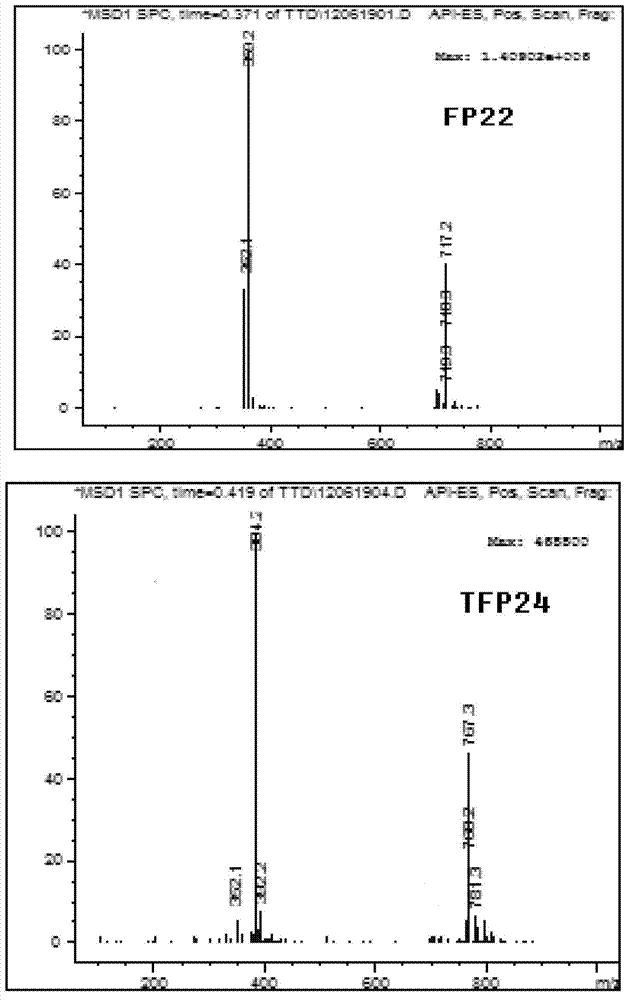
5-substituted tetrandrine compound and application thereof in preparing anticarcinogen sensitizer
A technology of tetrandrine and compounds, which can be applied in the fields of anti-tumor drugs, organic chemistry, drug combination, etc., can solve the problems that anti-tumor drugs are difficult to exert the expected effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1 synthesizes the compound shown in formula (I) according to the following scheme:
[0069]
[0070] Synthetic compound 1: put 1 equivalent of bromide tetrandrine, 2 equivalents of sodium azide, 10% equivalent of cuprous iodide, 30% equivalent of L-proline sodium and 30% equivalent of sodium hydroxide in a round bottom Add ethanol to the flask; react at 50°C for 4 hours, slowly add the reaction solution dropwise to 50ml of cold water while hot while stirring, a white solid precipitates out, and suction filters to obtain a white solid. TLC detection (or HPLC detection), refined in methanol.
[0071] Synthesis of compound 2: 1 equivalent of tetrandrine and 5 equivalents of cuprous cyanide were placed in a round bottom flask, and DMF was added. Replaced with inert gas for 3 times, heated to 135 degrees under the protection of inert gas (all substrates were dissolved), reacted for 5 hours, slowly added the reaction solution dropwise to 50ml of cold water whil...
Embodiment 2
[0081] Example 2 Chemotherapy Drug Doxorubicin (ADM) Effects on K562 and K562 / ADM, MCF-7 and MCF-7 / ADM Cell Proliferation
[0082] Take the K562 and K562 / ADM, MCF-7 and MCF-7 / ADM cells in the logarithmic growth phase and centrifuge, discard the supernatant, and adjust the cell number to 2×10 with RPMI1640 medium containing 10% calf serum. 4 / ml, add 96-well culture plate, add 100 μ l in every hole, each kind of cell is divided into 3 groups respectively: test group (different concentration chemotherapeutic drug ADM), negative control group (do not add medicine), blank control group (do not add cell only add culture medium). Place the 96-well plate at 37°C, 5% CO 2 In a saturated humidity incubator, after culturing for 48 hours, add 5g / L MTT20μl to each well, and after continuing to cultivate for 4 hours, add 100μl / well of triplex solution, culture over the liquid, select a wavelength of 570nm, measure the OD value on a microplate reader, and calculate Cell survival rate, IC5...
Embodiment 3
[0092] Example 3 Effect of Chemotherapeutic Drug Vincristine (VCR) on KB and KBV Cell Proliferation
[0093] Take the KB cells and KBV cells in the logarithmic growth phase and centrifuge, discard the supernatant, and adjust the cell number to 5×10 with DMEM medium containing 10% calf serum. 4 / ml, add 96-well culture plate, add 100 μ l in each hole, each kind of cell is divided into 3 groups respectively: test group (different concentration chemotherapeutic drug VCR), negative control group (do not add medicine), blank control group (do not add cell only add culture medium). Place the 96-well plate at 37°C, 5% CO 2 In a saturated humidity incubator, after culturing for 48 hours, add 5g / L MTT20μl to each well, and after continuing to cultivate for 4 hours, add 100μl / well of triplex solution, culture over the liquid, select a wavelength of 570nm, measure the OD value on a microplate reader, and calculate Cell survival rate, IC50 value of chemotherapeutic drugs, drug resistanc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com